AWA: Academic Writing at Auckland
A Research Methods Report helps the writer learn the experimental procedures and the ways research findings are made in that discipline (Nesi & Gardner, 2012, p. 153). The question to be investigated is often provided as part of the assignment, and there is usually less focus on existing research and much more on the methods and results of the writer's own research. An IMRD (Introduction, Methods, Results, Discussion) structure is often used. AWA Research Methods Reports include Experiment Reports, Field Reports and Lab Reports.
Title: Pleistocene Phylogeography for four Metrosideros (Myrtaceae) species in New Zealand using chloroplast DNA haplotypes
|
Copyright: Dhobasheni Newman
|
Description: Understanding repercussions of past events helps us to understand how taxa may fare in the future. Studying phylogeography provides a tool to understand this.
Warning: This paper cannot be copied and used in your own assignment; this is plagiarism. Copied sections will be identified by Turnitin and penalties will apply. Please refer to the University's Academic Integrity resource and policies on Academic Integrity and Copyright.
|
Writing features
|
Pleistocene Phylogeography for four Metrosideros (Myrtaceae) species in New Zealand using chloroplast DNA haplotypes
|
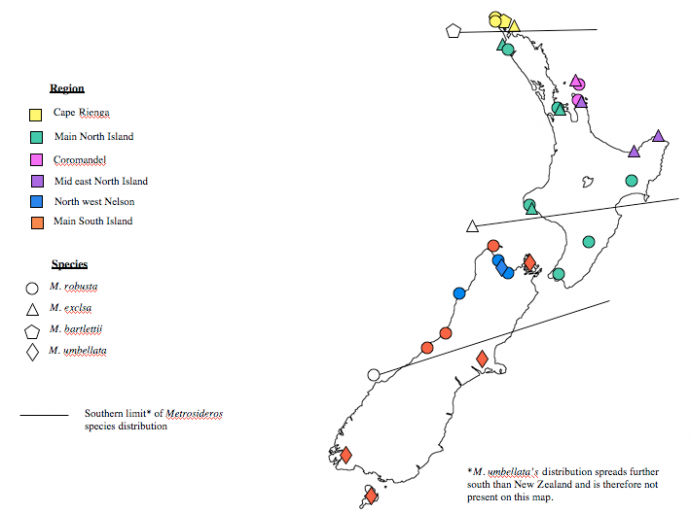
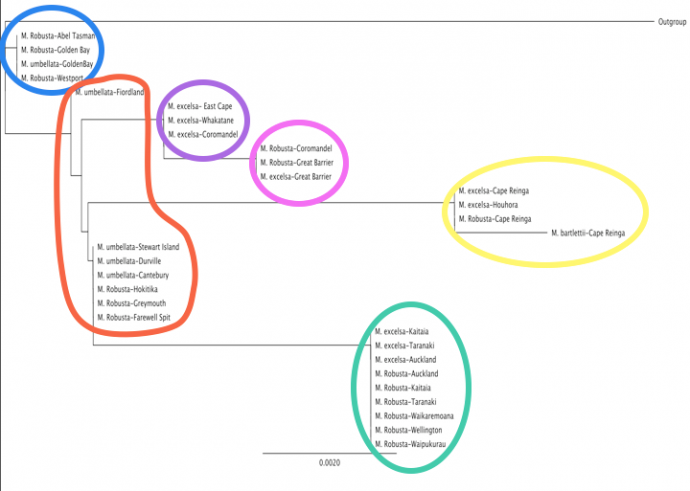
Abstract Understanding repercussions of past events helps us to understand how taxa may fare in the future. Studying phylogeography provides a tool to understand this. Few phylogeographical studies have been carried out in the southern hemisphere highlighting a need for this kind of research. Around 25,000 years ago the earth experienced the last glacial maximum where temperatures are considered to have been 4-6oC colder than current. It is thought that this event is likely to have wiped out many thermophilous plant species in New Zealand and seriously condensed the ranges of others. The species in the Metrosideros (Myrtaceae) genus survived this event most likely in areas of refugia which remained warm enough to support warm living plants during the glacial. Data from 30 samples of trnL-trnF locus of chloroplast DNA from Metrosideros excelsa, Metrosideros robusta, Metrosideros umbellata and Metrosideros bartlettii from all over the country were analysed. This resulted in 6 clear haplotype clusters and revealed three hotspots of diversity that can be identified as refugia. ____________________________________ Introduction Understanding past events and how they have affected distribution helps to enable us to understand and predict future fluctuations in distributions due to climate change (Shepherd, Perrie, & Brownsey, 2007). New Zealand is a long, remote archipelago of two major islands spanning a relatively broad mid latitude temperate zone (Gardner et al., 2004). This makes it ideal for studying glacial effects on phylogeography as it is remote enough from warmer northern tropical regions that Quaternary climatic oscillations are accentuated, having profound effects on frost sensitive biota (Gardner et al., 2004). Around 25,000 years ago the earth experienced the last glacial maximum where temperatures are considered to have been 4-6oC colder than current (Gardner et al., 2004; Mc Glone, 1985). It has been suggested that during glacial periods thermophilic plants that could not survive the decrease in temperature may have existed in small areas of warmer conditions or refuges (Petit et al., 2003). Many of these have been identified across the Northern Hemisphere (Gardner et al., 2004; Shepherd et al., 2007) and few have been identified for New Zealand (Mc Glone, 1985). The glacial refugia hypothesis suggests that before the last glacial maximum many taxa had broad distributions across much of the country. When ice and the range of cooler temperatures expanded ~25,000 years ago many plant taxa experienced a bottleneck event and were excluded from broad regions whilst some survived concentrated into warmer areas of refugia. Then with warming ~14,000 – 10,000 years ago the plants were able to spread and recolonise previously hostile areas (McGlone, 1985). Introgression is known to be widespread in the plant world (Gardner et al., 2004; Seehausen, 2004; Whitney, Ahern, Campbell, Albert, & King, 2010) and it is the process by which chloroplast genes can be swapped or shared between interspecific species (Seehausen, 2004). It occurs by the process of hybridisation between two species followed by multiple back crossings between the resulting progeny and either one or the other parent species (Whitney et al., 2010). Chloroplast and mitochondrial DNA are subject to uniparental inheritance (only inherited usually from the mother) and therefore with multiple back crossing with only one parental species there can be two results. Either the hybrid can regress back to the original parental chloroplast population or can swap genes, becoming essentially one species phenotypically and genetically in every way apart from chloroplast DNA which came from the other parental species not backcrossed with (Seehausen, 2004). The occurrence of introgression in plant communities often makes it difficult for taxonomists to form clear phylogenies to establish true evolutionary relationships as an individual may look like and essentially have all the genes of a particular species but possess the chloroplast genes of another (Petit et al., 2003). This however is very useful for establishing biogeographic relationships as distinct clusters form between species that have hybridised and then spread from a particular location enabling dispersal patterns to be tracked and refugia to be identified (Petit et al., 2003; Shepherd et al., 2007). Phylogeography (often in conjunction with palynology) has been very useful in identifying refugial sites and in some cases has been able to precisely infer postglacial colonisation routes. Much of this has been done in the Northern hemisphere with very little phylogeographical studies carried out in the southern hemisphere (Shepherd et al., 2007). The Metrosideros (Myrtaceae) genus is ideal for studying phylogeography as it has been described as exhibiting “classic” refugia and dispersal patterns (Gardner et al., 2004; Shepherd et al., 2007). The majority of the species found in New Zealand have ranges that span widely across the entire country (except Metrosideros bartlettii) and they are recognised as rapid prolific dispersers (Wright, Yong, Wichman, Dawson, & Gardner, 2001) allowing rapid recolonisation events and spatial reaction to have occurred between the last glacial maximum and today (Gardner et al., 2004). A subset of data on four Metrosideros species native to New Zealand from Gardner et al’s 2004 paper has been reanalysed and from that a scenario of refuge and post glacial dispersal of Metrosideros in New Zealand will be proposed and discussed. Methods The data used for this study is a subset consisting of only 30 samples of the 200 collected and analysed by Gardner et al (2004). The species considered for this study were Metrosideros excelsa, Metrosideros robusta, Metrosideros umbellata and Metrosideros bartlettii. kermadecensis was excluded from this analysis as it is not found on the New Zealand mainland. The section of DNA used in the analysis was trnL-trnF locus of cloroplast DNA and was analysed using the Sanger technique. For further detail on DNA analysis please refer to Gardner et al (2004). Data analysis Sections of Metrosideros DNA were identified and translated into lettered (ATCG) code and entered into the software program Geneious. Target sequences (trnL-trnF locus of cloroplast DNA) were identified, isolated and amplified using the software. Sections of DNA were then manually proofread to correct any incorrect pairings, insertions or deletions that may have occurred during the amplification process. The resulting sequences were then used to create a phylogenetic tree using the Geneious software rooted by an outgroup. The resulting clusters were then assigned colours and localities of corresponding samples were plotted onto a map. Results Locations and identities of the samples taken and analysed are observed in Figure 1. It can be seen that in areas of supposed refugia (areas of higher diversity suggested by a pilot carried out) more intense sampling was carried out as mentioned in Gardner et al (2004). The phylogram resulting from the analysis of the trnL-trnF sequences viewed in Figure 2 revealed 6 main clusters of Metrosideros, which when each sample location was plotted onto a map with a colour corresponding to cluster revealed 3 centres of high diversity (Fig. 1).
Figure 1: Map of New Zealand showing chloroplast haplotype locations for Metrosideros
Figure 2: Results of phylogenetic analysis of trnL-trnF sequences showing basepair differences between four species of Metrosideros. Coloured outlines refer to haplotype regions from Figure 1.
It can be seen in Figure 1 that bartlettii has a very narrow range confined to only the top reaches of the north island. M. excelsa across the northern and mid north island remaining mostly coastal. M.robusta has the greatest range across New Zealand ranging from the northern north island to the mid south island, remaining mostly to the west of the south island. M. umbellata’s range is only evident from the north to south of the south island. The blue and orange groupings were most closely related and found only on the south Island. Blue consisted of both M. umbellata and M. robusta and was confined to the North-west Nelson area (Fig. 2). Orange on the other hand consisted of the same species but was found to be widespread across the South Island. The purple, pink, green and yellow groupings were found only on the North Island. Purple was revealed to be the most related with the southern orange group, found only on the mid west coast of the North island and consisted only of M. excelsa. Pink is the next most closely related to purple and was restricted to the Coromandel area, consisting of both M.excelsa and M. robusta. Yellow and green were most derived with yellow slightly more than green. Green consisted of M. excelsa and M. robusta and was found to be widespread across most of the north island. Yellow was confined to the Cape Reinga region and consisted of M. robusta and M.excelsa also including M. bartlettii which was the most derived, sitting away from the rest in its grouping. Discussion The samples analysed formed 6 distinct phylogenetic groupings (Fig. 2) revealed to be based on geographic distribution (Fig. 1) and not species relations. Almost all clusters indicating genetic relatedness contained two or more species. This is possible due to the process of introgression. Hybrids are more likely to occur and persist in smaller populations affected by a bottleneck due to reduced competition from other non-hybrids, which would be present in a larger population (Mayr, 1996), therefore increasing the chance of subsequent introgressive back crossing which can lead to ‘gene swapping’ (Seehausen, 2004). It is this process which allows two phenotypically distinct species identified in the field, to be phylogenetically the same. There appears to have been broad hybridisation and introgression between robusta and M. excelsa in the North Island and M. robusta and M. umbellata in the South island. There is however no evidence in this data set of crosses between M. excelsa and M. umbellata or between M.bartlettii and any of the other species. There has been however documentation of natural hybridisation between M. excelsa and M. umbellata (Gardner et al., 2004) but the same is not true for M. bartlettii (Drummond, Keeling, Richardson, Gardner, & Wright, 2000). There are several locations of refugia across the North and South Islands suggested in other literature (Gardner et al., 2004; McGlone, 1985; Shepherd et al., 2007). These areas were subject to more intense sampling and have thus were revealed to be areas of higher haplotype diversity (Fig. 1). This provides evidence that they have indeed acted as refugia for Metrisideros. It has been suggested that during the last glacial temperatures would have been 4-6oC lower than today (Gardner et al., 2004; McGlone, 1985), this would have left few areas remaining warm enough for Metrosideros to survive in. There are three ‘hot spots’ of diversity observed (Fig. 1) in the Cape Reinga, Coromandel and North-west Nelson regions. These areas have previously been suggested as potential refugia (Gardner et al., 2004; Mc Glone, 1985). During the last glacial as temperatures dropped the warmest areas of the country would have been confined to the northern reaches of the islands. It was observed in Gardner et al (2004) that there were small regions in the tips of the northern reaches of both the North and South Islands where regional temperatures remained higher than the rest of the island. All but one (umbellata) of the four species of Metrisideros analysed in this study are known to be frost sensitive (Gardner et al., 2004) and so would not likely have survived in the cooler regions spanning most of the islands. This provides evidence for the glacial refugia hypothesis in New Zealand suggesting that when temperatures dropped only small populations in the slightly warmer north of the islands survived. Another interesting trend that can be observed is that there is one haplotype that dominates each of the islands. This can be explained by the concepts of succession and followed by competitive exclusion. As the temperature rose at the end of the glacial about 13,000 years ago (Mc Glone, 1985), conditions improved (became warmer) across greater area of the country. This allowed the survivors in refugia to recolonise a broader range. All Metrosideros are excellent dispersers and great primary sucessors but poor competitors (Wright et al., 2001). It is possible, due to hybridisation, one particular haplotype may have been better adapted to recolonise the surrounding (previously hostile) area (Mayr, 1996). Once colonised they spread to utilise the area available and competitive exclusion prevented other haplotypes from being able to colonise resulting in the observed pattern (Gardner et al., 2004). This pattern is characteristic of refuge and postglacial colonisation and has been observed elsewhere (Shepherd et al., 2007). It is however curious that it was the haplotype that was able to spread and not particular species characteristics that gave the initial successors an edge. This can be seen in Figure 1 where in the north island there is a clear mix of the species present, excelsa and M. robusta, possessing the same haplotype (green). Observed in the South Island orange haplotype however, is a distinction in the north-western distribution of M. robusta and the eastern and further south distribution of M.umbellata (Fig. 1). This is however quite likely explained by the difference in M. umbellatas ability to tolerate frost where as M. robusta is highly frost sensitive and also by the presence of the southern alps (Gardner et al., 2004).
Climatically stable regions in New Zealand have tended to retain greater proportions of older taxa (Mc Glone, 1985) therefore are likely to harbour highly endemic species as they are able to evolve for greater periods of time in these refuges. It is suggested that areas of refugia, providing stability and reduced variation resulting from a botttleneck due to die back in out of refugial regions, allow the accumulation of novel characteristics which would usually be selected against and bred out of a larger population (Mayr, 1996). Under this premise it can be postulated that the Cape Reinga refugia is likely to be the refugia existing through from prior to and then through the last glacial maximum as it contains and is the only known range of the most derived and therefore endemic species (Drummond et al., 2000) of Metrosideros considered in this analysis, bartlettii (Fig. 2). The other two locations of increased haplotype diversity are refugia from subsequent refuge and colonisation events (Gardner et al., 2004), the youngest of which being the North-west nelson refuge which can be considered the youngest as it has had the least amount of time to evolve endemism, hence the least derived haplotype (Mc Glone, 1985).
Conclusions Phylogeography and the analysis of trnL-trnF locus of cloroplast DNA provides a very useful tool for studying past refuge and dispersal events. Frost sensitive Metrosideros species were able to survive glacial maximum by existing in refugia. Prolonged periods inrefugia has lead to hybridisation and introgression between excelsa, M.robusta and M. umbellata and high endemism of these species also resulting an a highly derived recently evolved species M. bartlettii. Due to competitive exclusion following the subsequent recolonisation of the country when conditions began warming, single haplotypes dominate on each of the islands. Though three refugia can be recognised it is likely, based on phylogenetic relationships, that Cape Reinga was major refugia through glacial maximum and other two are result of subsequent refuge and colonisation events.
Reference list Drummond, R. S. M., Keeling, D. J., Richardson, T. E., Gardner, R. C., & Wright, S. D. (2000). Genetic analysis and conservation of 31 surviving individuals of a rare New Zealand tree, Metrosideros bartlettii (Myrtaceae). Molecular Ecology, 9, 1149-1157. Gardner, R. C., de Lange, P. J., Keeling, D. J., Bowala, T., Brown, H. A., & Wright, S. D. (2004). A late Quarternary phylogeography for Metrosideros (Myrtaceae) in New Zealand in ferred from chloloplast DNA haplotypes. Biological Journal of the Linnean Society, 83, 399-412. Mayr, E. (1996). What is a species and what is not? Philosophy of Science, 63(2), 262-277. Mc Glone, M. S. (1985). Plant biogeography and the late Cenozoic history of New Zealand. New Zealand Journal of Botany, 23, 723-249. Petit, R. J., Aguinagalde, I., de Beaulieu, J., Bittkau, C., Brewer, S., Cheddadi, R., et al. (2003). Glacial Refugia: Hotspots but not melting pots of genetic diversity. Science, 300, 1563-1565. Seehausen, O. (2004). Hybridization and adaptive radiation. Trends in Ecology and Evolution, 19(4). Shepherd, L. R., Perrie, L. R., & Brownsey, P. J. (2007). Fire and Ice:volcanic and glacial impacts on the phylogeography of New Zealand forrest fern Asplenium hookerianum. Molcular Ecology, 16, 4536-4549. Whitney, K. D., Ahern, J. R., Campbell, L. G., Albert, L. P., & King, M. S. (2010). Patterns of hybridization in plants. Perspectives in plant ecology, Evolution and Systematics, 12, 172-182. Wright, S. D., Yong, C. G., Wichman, S. R., Dawson, J. W., & Gardner, R. C. (2001). Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS+ETS). Journal of Biogeography, 28, 796-774.
|
|