AWA: Academic Writing at Auckland
A Research Methods Report helps the writer learn the experimental procedures and the ways research findings are made in that discipline (Nesi & Gardner, 2012, p. 153). The question to be investigated is often provided as part of the assignment, and there is usually less focus on existing research and much more on the methods and results of the writer's own research. An IMRD (Introduction, Methods, Results, Discussion) structure is often used. AWA Research Methods Reports include Experiment Reports, Field Reports and Lab Reports.
Title: Effects of temperature increase on heart rate in C. Japonica
|
Copyright: Megan Iminitoff
|
Description: Based on 3 hour lab - use relevant literature and write findings in the style of a scientific report, as if writing a paper for a scientific journal.
Warning: This paper cannot be copied and used in your own assignment; this is plagiarism. Copied sections will be identified by Turnitin and penalties will apply. Please refer to the University's Academic Integrity resource and policies on Academic Integrity and Copyright.
|
Writing features
|
Effects of temperature increase on heart rate in C. Japonica
|
Abstract Due to climate change, temperature is becoming an increasingly prominent concern for marine life and the heart is arguably the most susceptible organ to increases in temperature. It is important to be aware of the extent to which temperature increase affects invasive species in order to monitor their threat levels to native species. This study looks at the effects of thermal stress on the heart rate of C. japonica, an invasive species of paddle crab in New Zealand. Different parameters are examined including gender, previous experience of temperature increase, as well as starvation to assess wider physiological implications of thermal stress on the species. Increase in temperature was found to have a strong correlation with increase in heart rate. There were some observable trends showing variation in sensitivity within the previously mentioned sub-divisions, however none proved statistically significant. It can be said with some confidence that C. japonica displays some level of plasticity when it comes to dealing with thermal stress and this could prove problematic for native New Zealand species with regards to global warming.
Introduction Temperature is a major abiotic factor, which affects almost all aspects of physiology. This is especially pertinent in ectothermal animals as their body temperature is determined by the temperature of their environment (Guderley and St-Pierre, 2002). The heart in particular is very sensitive to increases in temperature and is generally the first to fail under thermal stress. Thus heart rate provides a good indicator of the effects of thermal variation on the stress levels of an animal (Iftikar, et. al., 2010). Additionally, because of the tight correlation between the circulatory system and ventilation, many factors such as temperature, which have an effect on heart rhythmicity also have an influence on oxygen consumption (McMahon, 1999). Due to the incredible sensitivity of physiological systems to temperature, all animals operate within a specialised thermal zone. Eurytherms in temperate environments like New Zealand typically have quite wide thermal ranges and are known to be able to shift their tolerance windows seasonally (Pörtner, 2002). Charybdis japonica is a eurythermal, invasive species of paddle crab inhabiting the waters of New Zealand. It displays sexual dimorphism, with differences in chelae and abdomen morphology, as well as size. Males grow larger than females and maximum size for C. japonicus is approximately 110mm (Kolpakov and Kolpakov, 2011). The ultimate temperature limits for this species are 4ºC – 34ºC in its native area, however in New Zealand waters it has been found in areas between 17ºC – 24ºC (Fowler, et. al., 2011). Thermal limits are an important environmental constraint on marine species. It is perhaps even more important when in relation to an invasive species such as C. japonica, as temperature can restrict how far the invasive species can spread and expand its new habitat (Fowler, 2011). New Zealand’s native paddle crab Ovalipes catharus is relatively sensitive to temperature changes. If temperature affects C. japonica to a lesser extent, it could become a problem for the native species as C. japonica could out compete O. catharus for resources and habitats (Iftikar, et. al., 2010). With respect to C. japonica, this study aims to determine how heart function varies with temperature and what this might mean for the animal both physiologically and ecologically.
Materials and Methods An individual paddle crab (Charybdis japonica) that had either been starved for a week or had been fed normally was placed into a polystyrene container, which was filled with 1 litre of room temperature seawater. Also inside the container was a bubbler that kept the water aerated. The crab was maintained at this temperature for approximately 20 minutes. A tripod was used to hold the crab still against the side of the polystyrene tub. Using a clamp mounted on a stand, a Doppler probe was positioned onto the crab’s shell above its heart. Headphones were plugged in to the Doppler, through which the heartbeat could be heard. A temperature probe was used to ascertain the initial temperature of the water in which the crab was immersed; this value was recorded in an Excel spreadsheet. Using a stopwatch, the number of heartbeats was counted for a 20 second period at the initial temperature; this number was then tripled to give heartbeats per minute. This was repeated three times in order to obtain an average number of heartbeats per minute and all values were recorded in the spreadsheet. Heated seawater was then added to the container until the temperature of the water had risen by 0.5ºC and as for the initial temperature, the heartbeat counts were repeated and recorded at this temperature. Using this method, heartbeats of the crab were measured and recorded at 0.5ºC increments until the water temperature reached 28ºC, or until the crab was too visibly agitated to continue. Once all the heartbeat measurements were completed, the crab was weighed; this value was recorded along with the gender of the crab. This method was completed for 15 crabs, and was then repeated two days later. Approximately half the crabs had been starved for a week, the other half had been fed regularly. The majority of the crabs that were used were the same individuals from the first day, however due to certain circumstances a few individuals needed to be replaced.
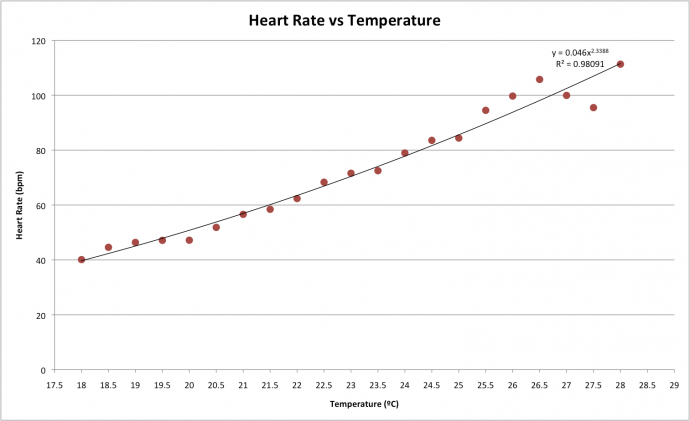
Results From the data that was collected, it is clear that there is a strong relationship between temperature and heart rate (figure 1). Figure 1 displays a positive relationship, with heart rate increasing as temperature increases. This trend conforms to an exponential trend-line with an R2 of 0.98 suggesting that the trend-line explains 98% of the variation in the data. Regression analysis provided a P-value of 7.919x10-16, displaying extremely strong evidence of a relationship between the two factors. Figure 1: Average heart rate in beats per minute against temperature of water in degrees Celsius for C. japonica. Trend line is an exponential curve. Analysis of averages for crabs from different days, different hunger states and different gender showed slight differences in sensitivity to temperature effects within these categories. Log transformations were used to convert the exponential data into linear data for comparable assessment and the results were graphed as Arrhenius plots (figure 2, 3, 4).
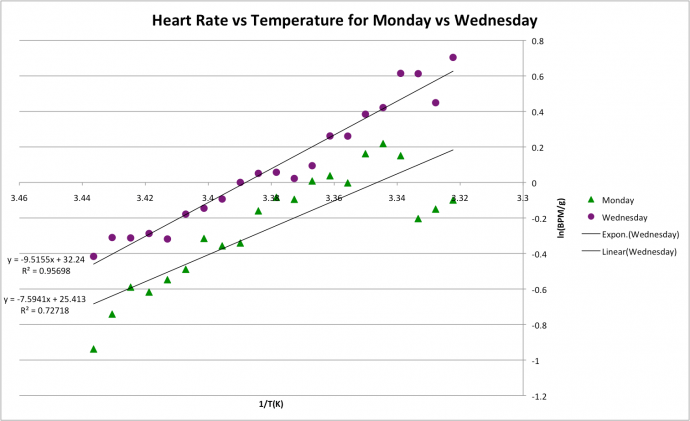
Figure 2 shows the Arrhenius plot for Monday and Wednesday crabs. The data conforms to the linear trend with R2 values of 0.73 and 0.96 respectively, indicating that the linear model is reasonable to use for prediction. The slopes for each group of crabs were -9.5 for the Wednesday group and -7.3 for the Monday group indicating a higher sensitivity to temperature in the Wednesday crabs. Figure 2: Arrhenius plot showing effects of temperature on heart rate in C. japonica. Crabs split into Monday stream and Wednesday stream.
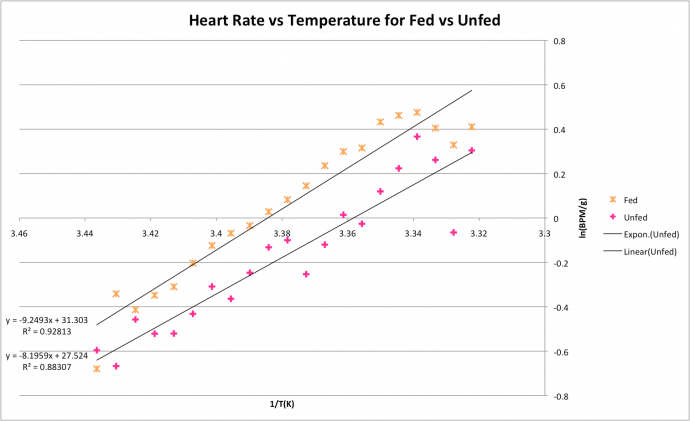
The Arrhenius plot comparing the fed and unfed crabs gave R2 values of 0.93 in the fed group and 0.88 in the unfed group (figure 3). This indicates the trend-line models the data well. Slopes indicate the fed crabs are marginally more sensitive to temperature change (slope of -9.3) than the unfed crabs (slope of -8.2).
Figure 3: Arrhenius plot showing effects of temperature on heart rate in C. japonica. Crabs split into fed condition and unfed condition.
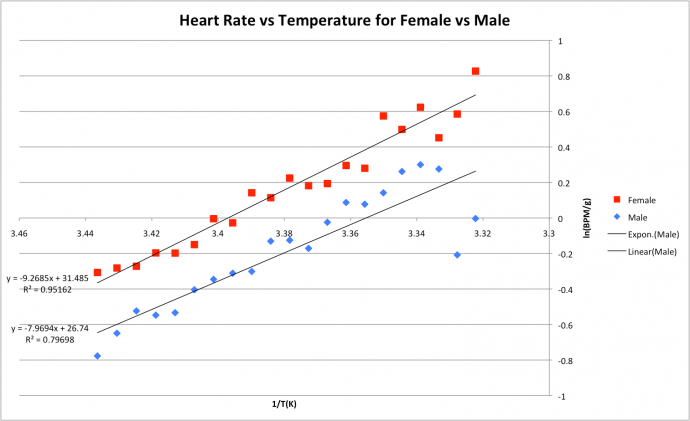
It was also found that male crabs were slightly less sensitive to change in temperature than the female crabs as the Arrhenius plot produced a slope of -7.97 for the males as opposed to the slope of -9.3 for females. The R2 were high for both datasets; 0.8 (male) and 0.95 (female). Figure 4: Arrhenius plot showing effects of temperature on heart rate in C. japonica. Crabs split by gender.
Discussion Heart rate is known to be extremely sensitive to almost any form of sensory stimulus (Florey and Kreibel, 1974). Temperature is no exception to this, as is exhibited by C. japonica. It is shown that the heart rate of C. japonica increases exponentially with increasing temperatures (figure 1). The reasons behind the increase in heart rate are beyond the scope of the experimental methods used. However, it has been suggested that acceleration of the heart rate with increasing temperature could be associated with an effect on the functioning of the heart musculature (Trueman and Lowe, 1971). Furthermore, in marine ectotherms, when the temperature exceeds the optimum range for the organism, a shift to partial anaerobiosis can occur for a limited time period (Sokolova, et. al., 2012). This shift typically occurs at the critical temperature for these organisms, which according to Sokolova, et. al. is also the temperature that delimits the population margins of marine species (2012). For C. japonica in New Zealand, the maximum temperature of its preferred range is approximately 24ºC (Fowler, et. al., 2011). Figure 1 displays a small drop in heart rate after 26.5ºC, similar to the trend that would be seen when the organism starts to shut down. The crabs were only taken to a temperature that caused them stress, rather than causing heart failure, therefore the thermal limit for this species is not defined by this study and no break point would be seen in the data. The drop in heart rate after 26.5ºC could be associated with the fact that the crabs were approaching their critical temperature and perhaps had made the switch to partial anaerobiosis. The crabs in this study were manipulated; half were fed and half were not. Furthermore there were two experimental groups, a naïve Monday stream that had not been exposed to the high temperatures and an experienced Wednesday stream. The individuals in the Wednesday group had been participants in the Monday stream and thus had undergone subjection to high temperatures. Lastly, there were both male and female crabs involved in this study. All of these factors could potentially affect response of heart rate to increased temperature, thus it was of use to look at them as separate groups. In general, starvation in crabs elicits a decrease in oxygen consumption. However, it has also been found to have an impact on temperature relationships (Marsden, et. al., 1973). The fed crabs in this study were shown to be slightly more sensitive to temperature change than the unfed crabs. This result was ascertained through the Arrhenius slopes seen in figure 3. Carbohydrate reserves are higher in well fed individuals, compared with the reserves of starved crabs. In a study done by Marsden, et. al., it was found that due to the higher carbohydrate reserved, fed crabs presented a more temperature-dependent metabolism than starved crabs (1973). This same phenomenon could account for the higher sensitivity seen by the fed C. japonica in contrast to the lower sensitivity in the unfed group. When a t-test was conducted for significance, there was however no significant difference found between the fed and unfed groups. This inconclusive result could be due to small sample size and/or limited time of starvation. The starved crabs were only unfed for one week, however crabs have been known to undergo elongated periods of starvation when limited resources are available and also when moulting (Matozzo, et. al., 2011). Arrhenius slopes for comparisons between Monday and Wednesday crabs similarly highlighted a marginal difference in sensitivity. The Monday crabs were found to be less sensitive to the temperature increase than the Wednesday crabs (figure 2). This is curious as it is expected that the experienced Wednesday crabs could have acclimatised somewhat to the change in environment. Contrastingly, this increase in sensitivity in the Wednesday stream could be due to the fact that when faced with thermal stress, ectotherms face a trade-off. The organisms must choose whether to maintain activity at a higher cost, or allow molecular processes to be affected by the temperature change (Guderley and St-Pierre, 2002). Having experienced an altered physiological state and perhaps still trying to recover, the Wednesday crabs’ sensitivity could thus be explained. It should be noted that as with the difference for the fed and unfed groups, there was no statistically significant basis for validation of this trend. Again based on Arrhenius slopes, a difference was seen between females, who were shown to be more sensitive to the temperature effects than males (figure 4). As C. japonica displays sexual dimorphism (Kolpakov and Kolpakov, 2011) it is reasonable to hypothesise that there could be a substantial difference in the impact of temperature on the heart rates of males versus females. However, the trend seen in the Arrhenius plots was once again not confirmed by a t-test, showing no true statistical difference between the sexes. Gender differences in response to thermal increases have been looked at in other studies done on marine arthropods. As aforementioned, oxygen consumption and heart rate are inherently linked, mitochondria require more oxygen at higher levels of stress. Studies have shown that oxygen consumption also increases in response to increases in temperature (Wachter and McMahon, 1996; Villarreal, 1990). In a study done on the effects of temperature increase on oxygen consumption in decapods there was no significant difference found between the sexes. Although similar to the results from this study, a trend was seen in the graphs (Villarreal, 1990).
Concluding Remarks From the results of the study, it is clear that there is an effect of temperature on the heart rate of C. japonica. However, the statistical inconclusiveness of any differences between fed, unfed, male, female, naïve and experienced crabs suggests a reasonable robustness of these crabs. Stress levels only seem to become significant for C. japonica above 26.5ºC, which is relatively warm for New Zealand waters. These elements in conjunction indicate that C. japonica has an effective strategy for dealing with temperature changes. This is a useful quality for a marine ectotherm to possess with regards to climate change as it leads to the belief that C. japonica would be able to adapt to higher temperatures if the necessity arises. As competition for O. catharus, this could pose a potential problem for the native species in the future.
References Florey, E., and Kriebel, M.E. (1974). The effects of temperature, anoxia and sensory stimulation on the heart rate of unrestrained crabs. Comparative Biochemistry and Physiology, 48A, 285-300. Fowler, A.E., (2011). Biological and ecological attributes of a population of the invasive Asian paddle crab, Charybdis japonica, in northeastern New Zealand (Thesis). University of Auckland, New Zealand. Fowler, A.E., Gerner, N.V., and Sewell, M.A. (2011). Temperature and salinity tolerances of Stage 1 zoeae predict possible range expansion of an introduced portunid crab, Charybdis japonica, in New Zealand. Biol Invasions. 13, 691-699. Guderley, H and St-Pierre, J. (2002). Going with the flow or life in the fast lane: contrasting mitochondrial responses to thermal change. Journal of Experimental Biology, 205, 2237-2249. Iftikar, F.I., MacDonald, J. and Hickey, A.J.R. (2010). Thermal limits of portunid crab heart mitochondria: Could more thermo-stable mitochondria advantage invasive species?. Journal of Experimental Marine Biology, 305, 232-239. Kolpakov, N.V. and Kolpakov, E.V. (2011). On the Biology of the Japanese Swimming Crab Charybdis japonica (Portunidae) in Waters of Primorye at the Northern Boundary of their Range. Russian Journal of Marine Biology, 37(7), 570-578. Marsden, I.D., Newell, R.C. and Ahsanullah, M. (1973). The effect of starvation on the metabolism of the shore crab, Carcinus maenas. Comparative Biochemistry and Physiology, 45A, 195-213. Matozzo, V., Gallo, C and Marin, M.G. (2011). Can starvation influence cellular and biochemical parameters in the crab Carcinus aestuarii? Marine Environmental Research, 71, 207-212. McMahon, B. (1999). Intrinsic and extrinsic influences on cardiac rhythms in crustaceans. Comparative Biochemistry and Physiology, 124A, 539-547. Pörtner, H.O. (2002). Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology, 132A, 739-761. Sokolova, I.M., Frederich, M., Bagwe, R., Lannig, G. and Sukhotin, A.A. (2012). Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Marine Environmental Research, 79, 1-15. Trueman, E.R., and Lowe, G.A. (1971). The effect of temperature and littoral exposure on the heart rate of a bivalve mollusc, Isognomum alatus, in tropical conditions. Comparative Biochemistry and Physiology, 38A, 555-564. Villarreal, H. (1990). Effect of temperature on oxygen consumption of the australian crayfish Cherax tenuimanus (Smith). Comparative Biochemistry and Physiology, 95A(1), 189-193. Wachter, B.D., McMahon, B.R. (1996). Temperature Effects on Heart Performance and Regional Hemolymph Flow in the Crab Cancer magister. Comparative Biochemistry and Physiology, 114A(1), 27-33.
|
|