AWA: Academic Writing at Auckland
A Case Study is one of a number of paper types (along with Problem Questions, Proposals and Designs) which identify and define a problem and recommend future actions. Case Studies are often used for real-life situations where the problem is complex and socio-economic contextual factors need to be considered as part of the recommendations (Nesi & Gardner, 2012, p. 188).
Title: Maketu Pā State of the Environment Report
|
Copyright: Pepijn Luiten
|
Description: Many agencies produce "State of the Environment Reports" to assess the status of the environment for a particular area or issue. The New Zealand Ministry for the Environment prepares State of the Environment reports on a national basis. Prepare a "State of the Environment Report" for Maketu Pa based on the data collected on the field trip. This will be a combination of an environmental 'report card' and issue-based 'snapshot' reports.
Warning: This paper cannot be copied and used in your own assignment; this is plagiarism. Copied sections will be identified by Turnitin and penalties will apply. Please refer to the University's Academic Integrity resource and policies on Academic Integrity and Copyright.
Maketu Pā State of the Environment Report
|
Maketu Pā State of the Environment Report
The principal purpose of state of the environment reporting should help New Zealanders make informed assessments regarding the current state, ongoing trends and primary threats regarding the environment, and create efficient management solutions to protecting its biodiversity. This report aims to identify and propose a key threat management strategy for the historic reserve, Maketu Pā , relevant to its identity as a cultural heritage site. We surveyed several habitat levels and potential ecosystem threats, using a range of standardised, repeatable monitoring methods. We assessed: bird species, Invasive weeds, pest species and freshwater habitat conditions. Overall we found that Maketu Pā displays a habitat symptomatic of lowland, broadleaf forest, that has been fragmented over generations of increased agricultural and urban land use. Key threats identified were ground-covering invasive weeds such as Tradescantia fluminensis and invasive mammalian pests, such as possums (Trichosurus Vulpecula) and Rats (Rattus sp). We concluded that targeted control of these key threats would result in multi-level benefits to other levels of the ecosystem, such as freshwater, plant, invertebrate and bird species. Overall the desired outcomes of the management strategy proposed are threefold: - Improved and sustained biodiversity - Accordance of cultural values - Increased support and engagement with the wider public.
Aotearoa has exceptional natural beauty. The environment is a tremendously important taonga, and sustains everything we as people depend on. Our personal health is interconnected to the ecosystems services offered by living organisms, studies show our mental health is dependant on the interactions we share with the environment (Anderson et al. 2000). We base our economy on our natural resources, Millions of tourists travel to New Zealand every year, to experience what we often take for granted. We share a deeply linked culture with the natural world, from a Maori perspective, Te Taiao (The natural environment) and everything within it, including tangata whenua (the people), are all connected. This means the state of the people is reflected by the state of the environment.
However, throughout New Zealand’s history, many ecosystems have been fragmented, reduced and exploited due to changes in land-use, fitting an ever-growing urban and agricultural agenda. Just over 30% of New Zealand is left forested, mostly inaccessible montane habitats, unappealing to human development. Lowland forests and wetlands are some of the most depleted ecosystems, and remnants of these systems are vulnerable to invasion of alien plant species and mammalian pests, which range across the majority of the country (Broome & Russel 2016). Environmental reporting highlights some of these key threats and allows the public and decision-makers to form opinions and make choices regarding these vulnerable systems.
Invasive pests are one of the biggest problems faced by conservationists, and nowadays New Zealand is considered the front-runner in mammalian pest management (Anderson et al. 2000; Bomford & O’Brien 1995). Mammalian pests cause pests cause significant impacts on biodiversity, predating on native flora and fauna, and displacing indigenous species through competition and disease (Bishop et al. 2015; Duncan et al. 2010). Furthermore, pests cost millions of dollars of output losses to primary production (Byrom et al. 2016).
New Zealand plays host to various alien plant species, many of which are invasive weeds, spreading throughout multiple ecosystems. The impact of these weed species is often prioritised less than mammalian pests, however these weeds can cause significant changes to composition and function of native ecosystems (Fowler et al. 2015). Various species can often change full scale ecosystem dynamics, such as altering soil nutrients, light regimes and supporting the growth of other alien species (Timmins & Williams 2006).
These threats are limiting factors on the success of our native fauna, which rely upon our native systems. New Zealand have a well known history of decline and extinction due to the introduction of anthroprogenic threats (Gillies et al. 2010). Therefore, substantial effort has gone towards conserving environments in which native bird species can recover. Accurate monitoring of bird species richness can indicate useful information regarding the health of a habitat.
These threats to native biodiversity are exacerbated by the fragmentation of indigenous forest. Catchment-scale land use has been a major factor affecting the health of our freshwater systems (Collier 1995). As agriculture increases in size and intensity, more freshwater systems are receiving greater inputs of nutrients, sediment and bacteria associated with agricultural catchements (Maxted et al. 2005). This can have severe impacts for the biodiversity of our waterways, with overall trends in freshwater systems across New Zealand increasing in nutrients like phosphorus and and nitrogen and decreasing in stream complexity and riparian vegetation.
These threats to biodiversity all apply to Maketu Pā , an Historic Reserve south of Auckland. Maketu Pā is a wai tapu (sacred) area to various Iwi/hapu, largely covered in mature, native forest representative of the former landscape, pre-human immigration. As a result, Maketu Pā has significant conservation value, being one of the remaining fragments in a country increasing in population growth, food demand and increased export trade.
This report will look at the state of the environment at Maketu Pā , and assess potential threats to the biodiversity value of the site. Considering the implications of Maketu Pā ’s cultural significance is paramount in this report, as it will not only enable alignment with the Maori view of the environment, but also may aid us in creating potential solutions to some key threats. The report will identify four areas and/or threats relevant to Maketu Pā , and investigate their significance. These areas include: Pest species, freshwater systems, invasive weed species and birds. Finally, a management strategy addressing the key threats identified will be proposed. This strategy will have clear outcomes aiming to minimise key threats, be relevant to the cultural context of the site and promote and encourage greater community engagement.
Maketu Pā is an historic reserve situated in East Ramarama, 40km south of Auckland central, New Zealand. On the south side of Maketu Stream, the reserve encompasses around 39ha, mostly lowland, mature, native forest. This forest stand has escaped the clearing of vegetation for agriculture, which has been an increasing theme of New Zealand land use in the last 800 years, and as a result contains a great diversity of native flora. Species found in Maketu Pā are typical of indigenous, podocarp-broadleaf forest, such as Dacrycarpus dacrydioides, Podocarpus totara, Beilschmiedia tawa and Dacrydium cupressinum. Maketu Pā exists as a ‘habitat patch’ within a greater network of indigenous vegetation patches spread across the South Auckland agricultural area. This network extends eastward of Maketu Pā, where land becomes less desirable for agriculture (Fig. 1). Larger fragmented patches continue for
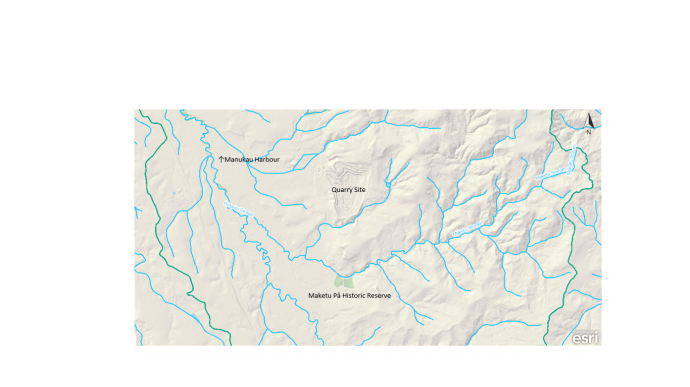
Figure 2. Catchment map of the Ramarama/Maketu Pā area. Map details the main topographical features, freshwater systems (blue lines) and catchment boarder (green lines). https://geomapspublic.aucklandcouncil.govt.nz/viewer/index.html
12.5km before reaching Hunua Ranges Regional Park, one of the largest remaining Podocarp-broadleaf forests. Covering over 40,000ha, the Hunua Ranges are a vital part of the remaining native forest in the North Island. Areas such as this have considerable conservation value, partly due to their endemism, but also due to their ability to sustain higher levels of biodiversity than other New Zealand landscapes (Anderson et al. 2000). Furthermore, the Hunua Ranges act as a corridor for native flora and fauna to spread across greater ecosystems. Hunua’s connectivity between the Hauraki islands, Waitakere Ranges and the Coromandel Peninsula, enables it to act as a source for greater spread into fragmented habitats, such as Maketu Pā. The Maketu stream starts east of Maketu Pā, flowing 6km west, passing through Maketu Pā before joining the Hingaia Stream and emptying into the south side of the Drury Creek in the Manukau Harbour (Fig. 2). These two streams cover the entire catchment in which Maketu Pā is located. The catchment is comprised of exotic pasture with smaller patches of urban development and fragmented forest. The Maketu Pā Reserve is one of the only riparian areas along the stream, with pasture land bordering most of the stream’s length, becoming increasingly urban as the stream connects to the harbour. Notably, a quarry exists less than a kilometre north of Maketu Pā, bordering one of the larger streams feeding into the Maketu River. Establishing Maketu Pā within the greater context of its surroundings is important. Both in its biodiversity and cultural value, Maketu Pā is influenced by the effect of its location. As a forest fragment, Maketu Pā’s placement within the larger indigenous forest network is important at a regional and national scale. Being close to the Hunua Ranges, Maketu Pā gains considerable conservation value as a nearby habitat sink for native dispersal, especially for avian and flora populations. Maketu Pā accessibility is also noteworthy. Whilst not influencing its biodiversity, or even influencing it negatively, it is important to consider Maketu Pā is directly off SH1 and within a short distance of Auckland, making one of the most accessible old growth stands.
4.1 Birds Methods To evaluate the state of the environment at Maketu Pā, we need concise, standardised methods to measure environmental variables. These methods also allow for contrast to other New Zealand environments.Five-minute bird counts (5MBC) are one standard method for bird estimates commonly used in forested ecosystems. 5MBC are conducted by recording all birds seen or heard within a stationary five-minute interval. Birds were not knowingly recorded multiple times and each count was at a 50m distance from other count sites. Three counts were conducted at the reserve site near the trail to the waterfall. In addition, the following weather variables were recorded: temperature, wind, other noise, sun, precipitation type and precipitation value. 5MBC estimates give a relative abundance and gives and index measure of commonly found bird species.
Results
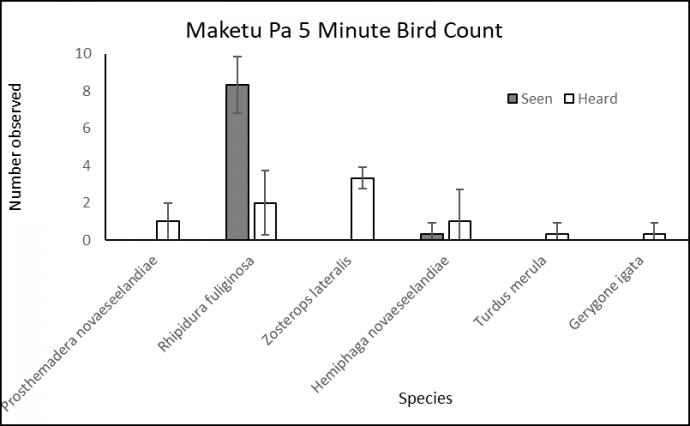
Figure 3. Results of two 5-minute bird counts, detailing several native and exotic bird species seen and heard at the Maketu Pā Historic Reserve. Discussion The dominance of Fantail and Silvereye species is consistent with studies addressing habitat structure and its effect on species coexistence (Fig. 3) (Barbaro et al. 2014; Norbury et al. 2014). The patterns we observed in community structure are reflected in several studies that predict a preference for indigenous forest shown by native bird species. Greene & Mortimer 2016 suggest species patterns such as high native species abundance (Fantail, Tui, Kererū) and low exotic abundance results from niche differences at a landscape scale, showing preferences for low and high exotic vegetation respectively (Barbaro et al. 2014; Fahrig et al. 1999). The notable exception being the Blackbird, found to show high prevalence in both native and exotic compositions. Partial niche filling and ecological generalism are characteristics of the Blackbird suggested to explain its high multiscale occurrence (Greene & Mortimer 2016; Williams 2006). Our survey revealed that Maketu Pā’s bird community is expected of native forest habitats with limited sampling (Minot & Moffat 1994). 4.2 Environmental weeds Methods
5 x 2m transect plots were conducted within the Maketu Pā site, recording invasive weeds. Walking counts were conducted lengthwise along the plot, recording the species and number of weeds within. This was repeated six times, three plots carried out in the forest interior and three at the forest edge (within 15m of zero canopy cover). Each transect was at minimum 30m from other transects.
Results
A total of 5 weedy, exotic species were recorded across all sites, 4 vasuclar plants and one saprobic fungi, Favolaschia calocera. Overall, weed abundance was low with all but one site having two or less weed species. Forest edge plots displayed a greater diversity and greater abundance of weed species compared to forest interior plots. Solanum nigrum (Black Nightshade) and F. calocera were the only two species detected in the interior plots, with Tradescantia fluminensis, Solanum mauritianum, S. nigrum and Cirsium vulgare all observed at forest edge sites.
Discussion Exotic weed species are commonly accepted to have impacts on native species and habitats. Whether through out-competing native species, reducing seedling recruitment, modifying soil structure or covering habitats and obscuring light, several weed species have been identified in New Zealand posing a serious threat to native biodiversity and potentially ecosystem function (Bellingham et al. 2001; Fowler et al. 2015; Lamoureaux et al. 2016). Often studies vary in methods, results, scale and conclusions when assessing the impact of weeds on native ecosystems, meaning there is no common consensus on the threat level of certain invasive species to native systems. However, studies have found certain weed species, such as T. fluminensis, being associated with dramatic declines in species richness in lowland forest. Forming a fast growing, dense mat-like growth on the forest floor T. fluminensis, effectively smothers all young vegetation and seedlings, blocking light and suppressing growth. Increases in T. fluminensis biomass is strongly associated with reductions in light availability, native species seedling recruitment and to nutrient availability (Lamoureaux et al. 2016). Generally, ground-covering weeds such as T. fluminensis successfully out-compete other species as light resources increase, meaning edge habitats or canopy gaps are susceptible sites for invasion. This reflects our results, discovering the greatest abundance of T. fluminensis at edge plots. Whilst found in relative low abundance in our survey, the potential threat posed by the spread of T. fluminensis to native species in more vulnerable habitats gives good reason to propose control methods, thus managing and protecting the future health of the forest. Due to several factors related to the location of Maketu Pā, prioritisation of T. fluminensis control at high light sites is required, where it is likely to exert the greatest impact (Lamoureaux et al. 2014). Firstly, Maketu Pā like other fragmented lowland forests, suffers from higher levels of edge area to interior area. Secondly, Maketu Pā’s proximity to urban environments allows for greater anthropogenic disturbances. These problems exacerbate the likelihood of weed invasion and success. Therefore, control of T. fluminensis at edge environments and preservation of canopy cover needs to be prioritised to reduce the risk of invasion to interior forest sites, thus enhancing the successful recruitment of native seedlings. 4.3 Introduced mammals Methods
Wax chew tags and ink tracking tunnels were left at several sites, including forest, pastoral and edge habitats around Maketu Pā for several days. Upon recollection, chew cards were inspected for damage and a key was used to estimate what species were present. Tunnels were also assessed, and species presence was recorded using a footprint key.
Results
Chew tags showed presence of Trichosurus vulpecula, Rattus sp. and Oryctolagus cuniculus. Tracking tunnels showed the presence of Rattus sp., Mus musculus, Erinaceus europaeus.
Discussion The presence of five introduced mammal species at Maketu Pā is expected given the habitat and species widespread prevalence in broadleaf-podocarp forest. Whilst all different, these species all pose a serious threat to the biodiversity and cultural health of the site (Bishop et al. 2015; Broome & Russel 2016). The occurrence of both Rats (Rattus sp.) and Mice (M. musculus) is expected of sites like Maketu Pā with strong edge effects and relative proximity to urban development (Anderson et al. 2000). Rats pose a serious conservation threat to native forest, their omnivorous diet causes predation upon various endemic species including birds, Weta and skinks, but also creates competition for key resources such as seeds (Bomford & O’Brien 1995; Gibbs 2009). The devastation of rats is also due to short generation times and high reproductive rate. After reductions to less than 5% in many New Zealand systems, Rats were found to have recovered to similar population sizes pre-reduction in less than four years (Byrom et al. 2016). Rats are known to have caused declines in populations of forest birds such as kākāriki (Cyanoramphus sp.), mōhua (Mohoua ochrocephala), North Island Robin (Petroica longipes), kokako (Callaeas sp.), kererū, and brown creeper (Mohoua novaeseelandiae) (Bishop et al. 2015). Brushtail Possums (T. Vulpecula) are viewed as ‘Enemy Number One’ when considering threats facing native biodiversity. Due to defoliation, tree species such as pōhutukawa (Metrosideros excelsa), kāmahi (Weinmannia racemosa), tawa (Beilschmiedia tawa) and more have suffered severe reductions in range, fecundity and health. These effects extend to changes in community level composition and structure (Bomford & O’Brien 1995). Similarly, Rabbits (O. cuniculus) can exert changes on flora composition, browsing on endemic vascular plants and spreading weed species seeds to native habitats. Possums are also bird predators and have been observed predating on all species mentioned in the bird presence data. Hedgehogs (Erinaceus europaeus) have also been identified as a conservation threat, showing they feed on a range of endemic bird eggs, invertebrates and lizards. One study found gut remains of individual Hedgehogs often showed the consumption of over 50 Weta in one night’s foraging. (Jones et al. 2005). 4.4 Water quality
Methods Environmental data and macro-invertebrate samples were collected from two sites at Maketu Pā . Both sites close in proximity (20m) but differed in watercourse characteristics, one being a low-flowing pond at the base of a short waterfall and the other being a short riffle, downstream of the pond. Physical features were determined visually, including previous rainfall measures, substrata type, shade level, sample microhabitat, water current speed and bank erosion level. Dissolved oxygen content, pH levels and Phosphate levels were measured by mixing sampled water with separate reagents before comparing formed colours to indicator charts. Water clarity was measured using magnetised clarity tubes and temperature readings taken from a spirit thermometer. Invertebrate samples were collected at steam edges using a 0.5mm mesh, triangular net and placing contents into observation trays. Presence of Invertebrate species was collected from these trays and converted into a Macro-invertebrate Community Index (MCI) soft bottom and hard bottom measure for the pond site and riffle site respectively (Stark & Maxted 2007). Results Strong similarities between both sites were seen across environmental and physical measurements. Physically, sites had similar levels of erosion, shade and air temperature. Additionally, water quality measures that were similar across both sites included air temperature, clarity, pH, dissolved oxygen and phosphorus levels. There was a 5°C decrease in water temperature from pond downstream to riffle. the riffle site also had a far greater MCI score. Table 1. Mean results from several freshwater measures taken from Maketu Pā Historic Reserve at two site: a low flowing pond and flowing riffle.
Discussion Waterways and the conditions surrounding them can be viewed at a number of scales. The physical and environmental characteristics of the immediate site, the conditions and intensity of use of the wider catchment, and also historic uses of the land all have impacts on freshwater habitats. Accessing environmental conditions at the immediate site, we can gain an appreciation of both the health of the waterway and how this could affect ecological communities. Our survey showed mixed conditions for a number of physical conditions as reported against Auckland Council’s Waicare standards. Both sites graded well for pH, dissolved oxygen levels and phosphorous levels. The pond site graded poorly for both water clarity and temperature. This is indicative of poorly shaded, low-flowing sampling sites, as riparian vegetation is known to influence both temperature and suspended sediment levels in streams (Collier 1995). These differences found between sites in proximity demonstrates that local variables can have significant affects on physical habitat quality.
Focusing wider, an assessment of the total catchment area shows majority of the land use both now and historically has been agriculture. Intensive agriculture can cause increased run off of nutrients, urine, waste and fertilisers containing nitrogen and phosphorus in to freshwater systems (Maxted et al. 2005). Other trends that impact waterways following the intensification of agriculture include increased soil compaction, greater run off and reduced riparian vegetation. These pressures negatively affect biodiversity, especially when compounded, and may cause serious threat to our freshwater species. The effects of these pressures are reflected in both sites MCI score.
A study across several sites in Auckland found a strong relationship between stream health and percentage of developed land (urban, agricultural) within catchments (Moore & Neale 2009; Stark & Maxted 2004). Streams located in highly developed catchments for use had low MCI scores, whereas the highest MCI scores tend to bed associated with mature native and exotic forest. Our sites displayed ‘poor’ and ‘fair’ quality stream, conforming to these findings.
5.1 Introduction The environment is complex, so gathering and analysing relevant information can be key to making environmental reporting purposeful. This is paramount when implementing management strategies to control biodiversity threats, as relevance should be the key criteria for when deciding upon conservation strategies. Whilst seemingly straight-forward, threat relevance is often hard to determine, as the amount we know about any given system is a function of how much study we have invested in said system, and the accuracy of those studies. Therefore greater attempts to understand our native forests and the both the existing and emerging threats they face is critical to management. However, lack of complete understanding should not equate to lack of conservation effort and strategy. Compromises must be made where scientific understanding comes up short, and indigenous and community knowledge in these situations that can be most valuable (Anderson et al. 2000). Location is important to bear in mind, as issues considered major threats in some ecosystems may be considered low value in others. Hence why large scale environmental reporting on a national scale such as Environment Aotearoa 2015 (Ministry for the Environment & Statistics New Zealand), can give us a broad overview of nation-wide trends, but inform us little of threats at local scales. Furthermore, these threats must be considered across levels and areas of the ecosystem. The consequences of confining a threat to one level e.g. waterways or forest birds, is a bias view of the entire issue. Efficient management strategies target issues that span multiple levels, resulting in benefits to multiple systems from one targeted approach. By using relevant information concerning the potential key threats to broadleaf-podocarp forests, we can identify key management actions for Maketu Pā that control and/or mitigate these threats. These actions must be pertinent to Maketu Pā’s smaller scale, and location within the broader environment. Preferably, these actions would tackle threats that span multiple ecosystem levels and confer benefits across different systems. Finally, management actions must be considered realistic given the time and resources affordable to Maketu Pā. 5.2 Strategy This State of the environment report proposes three desired outcomes from an implemented management strategy. Firstly, the control and mitigation of biodiversity threats, which is intrinsically linked to the conservation of the sites ecological health. Secondly, maintaining the cultural well-being of the site by protecting taonga and/or culturally significant resources, aligning with the goals of the ‘Maketu Pā Historic Reserve Goals and Recommendations’ proposed by the Whatapaka Marae Reservation Trust Board. Finally, improving public awareness and support for the preservation of Maketu Pā. 5.3 Threats While we do not fully understand to what extent, it is clear that exotic weeds pose a threat to native ecosystems. This especially applies to sites like Maketu Pā, being small and fragmented with high edge:interior ratios (Fowler et al. 2015). However, changes in our thinking towards exotic species in our native systems could provide several benefits with regards to our conservation efforts. The idea of a mixed community of endemic and exotic species does not seem representative of a ‘native system’ in the minds of many New Zealanders, including conservation parties. Nevertheless, ecosystems are dynamic and ideas of preserving a ‘pre-anthropogenic’ forest community goes against the ever changing nature of long term forest succession (Anderson et al. 2000). Intensively managed reserves containing a few endangered endemic species will continue to be a part of New Zealand's conservation effort, but the remainder and majority of New Zealand’s forested ecosystems are continuing to change. Therefore, by assessing exotic weeds based on their overall threat to the biodiversity of the entire ecosystem, we can focus our efforts on few key species that pose serious threat, and regard species that pose little or no threat as a part of an ever-changing ‘native’ ecosystem. As stated earlier, T. fluminensis is such a species posing serious threat to biodiversity and is a key weed to be controlled under a Maketu Pā management strategy. The devastating impact of ground cover weeds such as T. fluminensis have shown to reduce species richness, especially in endemic systems - compromising forest health. However, high growth levels of T. fluminensis are limited to high light environments (Lamoureaux et al. 2014). Therefore, sensible management would prioritise an initial blanket eradication from the site, following guidelines established by the Department of Conservation’s ‘Methods for the Control of Wandering Jew’ (Lovelock & Ogle 1989), followed by continual removal of T. fluminensis from edge areas. Additionally, preventing reductions to canopy cover in the interior forest (potentially caused by defoliation by possum and rat browsing) would limit opportunities for invasion (Lamoureaux et al. 2016). This leads into the second key threat. Pest management is expensive, and the amount of land we can exert control is but a fraction of the area where these species occur. Prioritising the control of pest species is often complex both in theory and practice. Deciding what pest poses the greatest threat to biodiversity, and will result in the greatest conservation value can vary from site to site (Brown et al. 2015). Methods need to be reliably and repeatably effective to reduce pest numbers to obtain significant benefit. Rats and brush-tail possums are two invasive pests in which control would offer the significant value. Control must be multi-approach, with growing recognition that control of one species can have variable effects on the abundance of another non-target pest (Broome & Russel 2016; Brown et al. 2015). High intensity ground control, including baiting and trapping, with 5-6 year returns for the targeted removal of possums and rats is a realistic strategy given the information presented. This conclusion is based on a number of factors. Firstly, ‘High-intensity’, defined as a level that equates to the intensity of control under the ‘Mainland Island’ programme proposed by the Department of Conservation, has been found to produce considerably greater results regarding the reduction in pest abundance, comparable to other lower levels of control. Secondly, Whilst potentially more effective, aerial control (poisoning) is unfeasible given the cost, proximity to urban development, small scale of the forest and ease of public access. Finally, ground control methods are most accessible to the public, which improves its capacity for greater collective initiative. The reduction of pest species into Maketu Pā should not only provide immediate ecological benefits, but also produce multi-level effects, improving habitat health across multiple scales (Bishop et al. 2015). Reduction in mammalian predators would minimise the threats to native bird, reptile, invertebrate and tree species (Barbaro et al. 2014; Gibbs 2009). Decreases in defoliation would retain greater canopy cover, minimising the threat of weed invasion to the forest (Payton 2000). Additionally, reduced defoliation would improve soil retention as there would be greater species survivorship resulting in decreased sediment load into the freshwater system (Collier 1995; Maxted et al. 2005).
5.4 Cultural Maketu Pā is a culturally relevant site with connections to several Iwi/Hapu. The Maketu Pā Reserve is wahi tapu (sacred) to Te Waiohua descendants including Nga-ti Pou, Nga-ti Tai, Nga-ti Tamaoho, Te Akitai, Nga-oho and Nga-ti Koheriki. The reserves is under management by the Whatapaka Marae, who proposed the ‘Maketu Pā Historic Reserve Goals and Recommendations’ in 2011. This document outlines several desired outcomes to ensure kaitiakitanga of their taonga. Building upon the context of that document, this management strategy and focuses more on biodiversity protection. It is also recommended integrating different tangata whenua into the responsibilities of carrying out this management strategy will allow individuals to develop relevant conservation and pest management skills. In turn, making communication between tangata whenua related to the rohe (region) and other interested conservation agencies easier, providing a base for engagement into further conservation (Anderson et al. 2000). 5.5 Community This management strategy also recommends collaboration with a broader range of industries, agencies, education centres and community groups/members with intersecting interests. The last of the desired outcomes proposed by this strategy is greater public awareness and support. By sharing resources and and decision making, the major outcome of reducing targeted threats to Maketu Pā will become much more realistic. Collective endeavours will confer a number of benefits, such as adding funding and ensuring it is coordination and realism, greater publicity and encouragement for similar endeavours, increased expertise in effective threat management and potential education of the general public. 5.6 Conclusions If this management plan was adopted, taking similar measures outlined in this report annually on a more comprehensive scale would be recommended. Comparing the results obtained to other systems both managed and unmanaged would present an idea of the progress which Maketu Pā’s environment. In summary, this report proposes an achievable management plan to attain three desired outcomes. Controlling key biodiversity threats T. fluminensis and mammalian predators. Management actions aim to reduce these threats to insignificant numbers, and also prevent further establishment, thus protecting Maketu Pā ’s biodiversity and recognising it as a site of ecological importance. Furthermore, this plan is aware and adheres to the cultural significance of Maketu Pā , promoting kaitiakitanga of our native environments. Finally, this plan hopes to build public awareness, support and collaboration for greater conservation efforts, here and elsewhere.
References Anderson S, Clout M, Craig J, Creese B, Mitchell N, Ogden J, Roberts M, Ussher, G 2000. Conservation Issues in New Zealand. Annual Review of Ecology Systems 31: 61-78. Barbaro L, Barnagaud JY, Brockerhoff EG, Deconchat M, Papaix J 2014. Habitat filtering by landscape and local composition in native and exotic New Zealand birds. Ecological Society of America 95: 78-87. Bellingham PJ, Burrows LE, Wiser SK 2001. Managing biodiversity information: development of New Zealand’s National Vegetation Survey databank. New Zealand Journal of Ecology 25: 1-17. Bishop C, Didham RK, Innes J, Khin J, Landers T, Ruffel J 2015. Using pest monitoring data to inform the location and intensity of invasive –species control in New Zealand. Biological Conservation 191: 640-649. Bomford M, O’Brien P 1995. Eradication or Control for vertebrate pests? Wildlife Society Bulletin 23: 249-255. Broome KG, Russel JC 2016. Fifty years of rodent eradications in New Zealand: another decade of advances. New Zealand Journal of Ecology 40: 197-204. Brown PH, Byrom AE, Innes JG, Russel RC 2015. Predator-free New Zealand: Conservation Country. Bioscience 65: 520-525. Byrom AE, Choquenot D, Forsyth DM, Nugent G, Parkes JP, Pech RP, Warburton B 2016. Past, present and two potential futures for managing New Zealand’s mammalian pests. New Zealand Journal of Ecology 41: 151-161. Collier KJ 1995. Environmental factors affecting the taxonomic composition of aquatic macroinvertebrate communities in lowland waterways of Northland, New Zealand New Zealand Journal of Marine and Freshwater Research 29: 453-465. Duncan R, Holland P, Nugent G, Sweetapple P, Whitford J 2010. Effect of one-hit control on the density of possums (Trichosurus vulpecula) and their impacts on native forest. Department of Conservation, Wellington. Fahrig L, Merriam G, Trzcinki MK 1999. Independent Effects of forest cover and fragmentation on the distribution of forest breeding birds. Ecological Applications 9: 586-593. Fowler SV, Hayes L, Hill RL, Paynter Q 2015. Factors affecting the cost of weed biocontrol programs in New Zealand. Biological Conservation 80: 119-127. Gibbs GW 2009. The end of an 80-million year experiment: a review of evidence describing the impact of introduced rodents on New Zealand’s ‘mammal-free’ invertebrate fauna. Biological Invasions 11: 1587-1593. Gillies C, Innes J, Kelly D, Overton JM, 2010. Predation and other factors currently limiting New Zealand forest birds. New Zealand Journal of Ecology 31: 86-114. Greene TC, Mortimer JA 2016. Investigating bird calls identification uncertainty using data from processed audio recordings. New Zealand Journal of Ecology 41: 126-133. Jones C, Moss K, Sanders M 2005. Diet of hedgehogs (Erinaceus europaeus) in the upper Waitaki Basin, New Zealand: Implications for Conservation. New Zealand Journal of Ecology 29: 29-35. Lamoureaux SL, McAlpine KG, Timmins SM, Wotton DM 2016. Native woody plant recruitment in lowland forests invaded by non-native ground cover weeds and mammals. New Zealand Journal of Ecology 41: 65-73. Lamoureaux SL, McAlpine KG, Westbrooke I 2014. Ecological impacts of ground cover weeds in New Zealand lowland forests. New Zealand Journal of Ecology 39: 50-60. Lovelock B, Ogle C 1989. Methods for the control of Wandering Jew (Tradescantia Fluminesis) at ‘Rangitawa’ Rangitikei District. Wanganui Conservancy, Department of Conservation, Wellington, New Zealand. Maxted JR, McCready CH, Scarsbrook MR 2005. Effects of small ponds on stream water quality and macroinvertebrate communities. New Zealand Journal of Marine and Freshwater Research 39: 1069-1084. Ministry for the Environment & Statistics New Zealand 2015. New Zealand’s Environmental Reporting. Series: Environment Aotearoa 2015. Available from www.mfe.govt.nz and www.stats.govt.nz. Ministry for the Environment & Stats NZ 2017. New Zealand’s Environmental Reporting Series: Our freshwater 2017. Retrieved from www.mfe.govt.nz and www.stats.govt.nz. Minot EO, Moffat M 1994. Distribution and abundance of forest birds in the Ruamahanga Ecological area, North Island, New Zealand. New Zealand Journal of Zoology 21: 135-150. Moore S, Neale MW 2009. Freshwater invertebrate monitoring: 2003-2007 analysis and evaluation. Prepared by Landcare Research and Auckland Regional Council for Auckland Regional Council. Norbury G, Walker S, Wilson DJ 2014. How does woody succession affect population densities of passerine birds in New Zealand drylands? New Zealand Journal of Ecology 38: 257-267. Payton I 2000. Damage to native forests. The brush-tail possum: biology, impact and management of an introduced marsupial. Manaaki Whenua Press, Lincoln, New Zealand 111-125. Stark JD, Maxted JR 2004. Macroinvertebrate community indices for Auckland’s soft-bottomed streams and applications to SOE reporting Prepared for Auckland Regional Council. Cawthron Report No. 970. Nelson Cawthron Institute. 303: 59. Stark JD, Maxted JR 2007. A biotic index for New Zealand’s soft-bottomed streams. NZJ Freshwater Research 41: 43-61. Timmins SM, Williams PA 1991. Weed numbers in New Zealand’s Forest and Scrub Reserves. New Zealand Journal of Ecology 15: 153-162. Williams PA 2006. The role of blackbirds (Turdus merula) in weed invasion New Zealand. New Zealand Journal of Ecology 30: 285-291. Wellington, New Zealand, New Zealand Parliamentary Commissioner for the Environment 2016. The state of New Zealand’s environment: Commentary by the Parliamentary Commissioner for the Environment on Environment Aotearoa 2015. P.52.
|