AWA: Academic Writing at Auckland
A Case Study is one of a number of paper types (along with Problem Questions, Proposals and Designs) which identify and define a problem and recommend future actions. Case Studies are often used for real-life situations where the problem is complex and socio-economic contextual factors need to be considered as part of the recommendations (Nesi & Gardner, 2012, p. 188).
Title: Invasive species report: Asian Honey Bee
|
Copyright: Stephanie Morton
|
Description: The purpose of the species risk assessment is to:
• compile all known information about the species relevant to its status as a potential invasive species in New Zealand;
• make a judgment (with justifications) as to the pest status of the species;
• compile known information about control/management methods
• identify knowledge gaps;
• make research recommendations.
Methodology: Use primary literature, grey literature, information from the web (referencing url and date accessed), and personal communications from national and international experts (using email and listservers if necessary). Examples: Hymenoptera Name Server, Plants Database (Landcare Research databases), weed control databases, Google search "spp. name + pest/weed + control"
Warning: This paper cannot be copied and used in your own assignment; this is plagiarism. Copied sections will be identified by Turnitin and penalties will apply. Please refer to the University's Academic Integrity resource and policies on Academic Integrity and Copyright.
Invasive species report: Asian Honey Bee
Stephanie MortonSpecies risk assessmentApis cerana (Asian honey bee)
Contents
1.0 Taxonomy
1.1 Full Scientific Name pg 2 1.2 Taxonomy pg 2 1.3 Taxonomic Notes pg 2 1.4 Common Names pg 2 1.5 Description/Identification pg 2 1.6 Similar Species pg 3
2.0 Range 2.1 Native Distribution and Spread pg 4 2.2 Potential Range pg 4
3.0 Biological Characteristics and Uses
3.1 Reproduction and Fecundity pg 5 3.2 Behavioural Patterns pg 5 3.3 Habitat pg 5 3.4 Foraging and Feeding pg 5 3.5 Ecological Role and Uses in Native Range pg 5
4.0 Potential Impact
4.1 Native Species Diversity and Competition pg 6 4.2 Ecosystem Change pg 6 4.3 New Zealand Honey Bee Competition pg 6 4.4 New Zealand Honey Bee Disease Risk pg 7 4.5 Human Health and Uses pg 7
5.0 Control Technologies and Strategies
5.1 Potential Eradication pg 8 5.2 Monitoring pg 8 5.3 Baiting and Control pg 9 5.4 Biological control pg 9
6.0 Knowledge Gaps and Research Recommendations
6.1 Knowledge Gaps pg 9 6.2 Research Recommendations pg 9 6.3 Justification for Inclusion pg 10 6.4 Justification Against Inclusion pg 10 6.5 Conclusion pg 10
7.0 References pg 11-12
1.0 Taxonomy
1.1 Full Scientific Name Apis cerana (Hepburn and Radloff, 2011)
1.2 Taxonomy Kingdom: Animalia Phylum: Arthropoda Class: Insecta Order: Hymenoptera Suborder: Apocrita Family: Apidae Subfamily: Apinae Genus: Apis Species: cerana
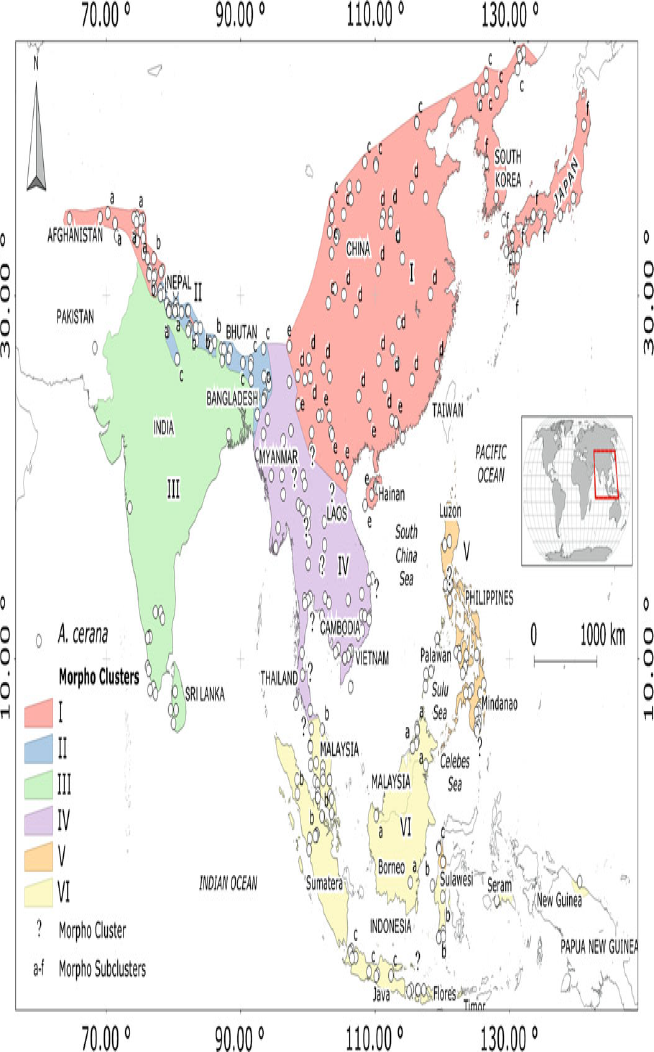
1.3 Taxonomic Notes There are 6 distinct morphoclusters defined within A. cerana (see figure 1) (Tan, Qu, Wang, Liu & Engel, 2015): Northern A. cerana (containing six sub-clusters), Himalayan A. cerana (containing two sub-clusters), Indian plains A. cerana, Indo-Chinese A. cerana, Philippine A. cerana (Tropical wet climates type, containing three sub-clusters), and Indo-Malayan A. cerana (Tropical wet climates type, containing three sub-clusters) (Hepburn and Radloff, 2011; Tan et al., 2015). These morphoclusters represent one species but display biological and ecological plasticity between regions. For the purposes of this report we will be examining all the Apis cerana morphoclusters collectively as incursion may originate from any one of these Asian populations.
Figure 1. Geographic distribution of Apis cerana morphoclusters. Image sourced from Hepburn and Radloff, 2011.
1.4 Common Names Asian honey bee, Asiatic honey bee, Asian hive bee, Indian honeybee, Indian bee, Chinese bee, Mee bee, Eastern honey bee, Fly Bee (Koetz, 2013).
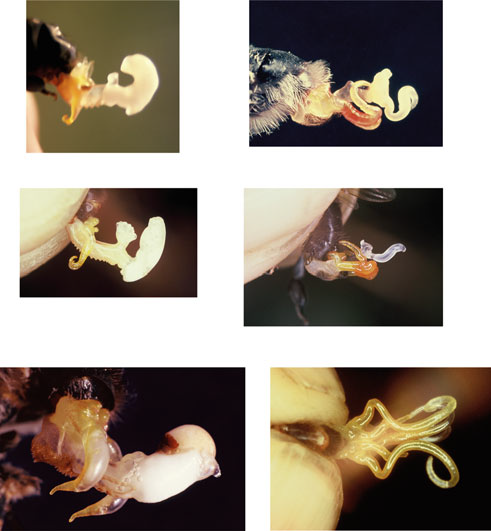
1.5 Description/Identification Apis cerana are social nesting bees with three distinct morphotypes; queens, drones and workers (see figure 3 and 4). They are typically cavity nesting with colony size ranging between 1,400-34,000 bees (Koetz, 2013). Apis cerana are smaller than A. mellifera growing up to 10mm in length, however size varies across their native range (tropical sub-species are morphologically smaller than the sub-tropical varieties) (Koetz, 2013). Workers have yellow/dark brown and black stripes that remain evenly spread along the whole abdomen, unlike A. mellifera whose stripes vary in width and get darker toward the end of the abdomen (see figure 2) (Queensland Government Department of Agriculture and Fisheries, 2016). Drones and queens are darker than workers (see Figure 3 and 4). Apis cerana are less hairy in general, and the base of the wings look blacker and shinier than A. mellifera (see Figure 2) (Queensland Government Department of Agriculture and Fisheries, 2016). Apis cerana has faster, more erratic flight patterns and are active earlier and later in the day (Koetz, 2013; Queensland Government Department of Agriculture and Fisheries, 2016). The endophallis of the males is also distinctively different from all other Apis species and can be viewed in Figure 5. The Australian government has released a comprehensive identification chart for A. cerana in the ‘NAQS Asian honey bee floral surveillance manual’ that we recommend for use. You can find this at: http://www.agriculture.gov.au/SiteCollectionDocuments/pests-diseases-weeds/bees/naqs-asian-honey-bee-floral-surveillance-manual.pdf (released under Creative Commons Attribution 3.0 Australia Licence).
Figure 2. Left- Apis mellifera (European honey bee) worker, Right- Apis cerana (Asian honey bee) worker. Photograph by Paul Zborowski
Figure 3. Morphological variation between queen (reproductive female) A. cerana and workers. Photograph by Azman, CC BY-SA-3.0.
Figure 4. Five species of Asian honey bee drones (below) and workers (above). From left to right A. koschevnikovi, A. nuluensis, A. cerana (in middle), A. andreniformis and A. dorsata. © Springer-Verlag Berlin Heidelberg 2011.
Figure 5. Endophallus of A. cerana (male). © Springer-Verlag Berlin Heidelberg 2011.
1.6 Similar Species The most notable species currently present in New Zealand is the European honey bee (A. mellifera). This was introduced to New Zealand in 1839 for crop pollination and honey production (Early, 2007b). Apis cerana shares a very similar behavioural traits and niche to A. mellifera (Dyer and Seeley, 1991). New Zealand has three genus of native bee species; Leioproctus bees (18 species), Hylaeus bees (7 species), and Lasioglossum (4 species). All native species are solitary, do not produce honey in commercial quantities, and are mostly ground dwelling (Early, 2007a). There are also four species of bumble bee (Bombus), introduced to New Zealand for agricultural pollination services (Early, 2007a).
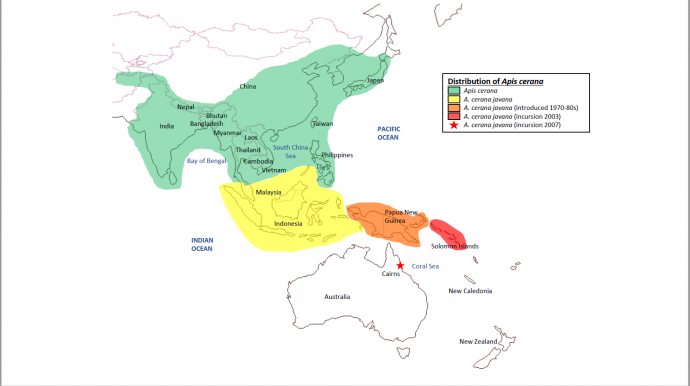
2.0 Range 2.1 Native Distribution and Spread Apis cerana display a tremendous amount of plasticity in their biology and ecology. Their native distribution extends across Asia from Afghanistan to Japan and down through South East Asia (see Figure 6). More recently (1970-80s) A. cerana was introduced into Papua New Guinea. From there A. cerana invaded the Solomon Islands in 2003, and established in Cairns, Australia in 2007 (Koetz, 2013). Apis cerana is showing a downwards migration and will most likely spread through Australia (Heersink, Caley, Paini and Barry, 2016). Spread is enabled through shipping ports. Containers and boats provide many cavities for them to nest. It has yet to reach New Zealand borders.
Figure 6. Current species distribution of Apis cerana including most recent spread into Australia.
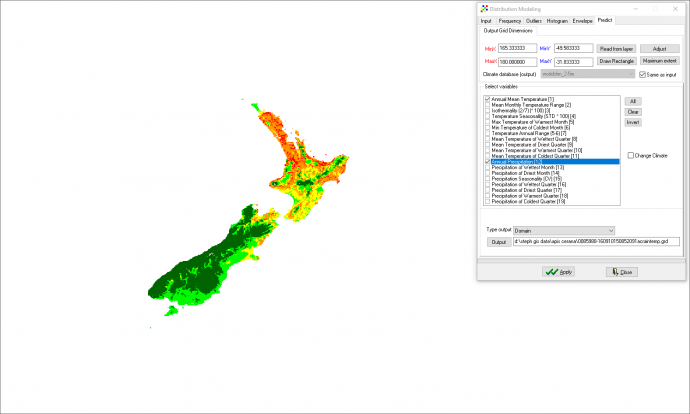
2.2 Potential Range Climate modelling suggests that there is an extremely favourable climate (suitability >90%) for A. cerana to establish throughout the North Island and the northern and eastern coast of the South Island (see Figure 7). The areas of lower probability of establishment are the western and central South Island, and at higher altitudes (Mt Taranaki, Mt Ruapehu, Hutarau ranges, the Southern Alps). The most likely incursion sites in New Zealand will be the through the ports (Heersink et al., 2016). This is of great concern as our two major ports (Auckland and Tauranga) are located in areas with 98-100% climate suitability for A. cerana establishment. Diet/habitat potential range can be assumed to match this climate model as A. cerana diet and habitat preference are comparable to A. mellifera which can be found throughout the same regions of New Zealand. It is to be expected that A. cerana will be able to follow similar distribution patterns to A. mellifera should it establish in New Zealand. Figure 7. Apis cerana climate suitability in New Zealand based on annual mean temperature and precipitation comparability using the Grower distance statistic. Values >90 are considered suitable for establishment.
Distribution map is created through DIVA-GIS software, Creation Date: Saturday, April 29, 2017 9:11:31 AM CEST. Records included: 1309 records from 13 published datasets, Filter used: TaxonKey: Apis cerana Fabricius, 1793. This download can always be viewed on http://www.gbif.org/occurrence/download/0085988-160910150852091
3.0 Biological Characteristics and Uses 3.1 Reproduction and Fecundity Apis cerana queens mate at 5-6 days during one nuptial flight with many drones which die shortly after (Hepburn and Radloff, 2011). Development time from oviposition to hatching takes 20 days for workers, 23-24 days for drones, and 14-15 days for queens (Punchihewa, 1994 as cited in Hepburn and Radloff, 2011). Colony size ranges from 1,400-34,000 bees, but on average are around 6,884-14,745 individuals (Koetz, 2013). The mating season is linked to blooming cycles and coincides with A. mellifera mating (Koetz, 2013). Apis cerana and A. mellifera have similar pheromones and are capable of interbreeding naturally (Koetz, 2013). However, interbreeding will not produce viable offspring, and usually leads to colony loss (Hepburn and Radloff, 2011). Interspecific competition occurs in areas of co-habitation. The higher density species drones will out-compete the lower density species for queen mating’s (Koetz, 2013). Apis cerana has a competitive advantage during species interbreeding. Worker bees (in the absence of a viable queen) can reproduce by thelytoky. Around 2% of eggs laid by workers will develop into females which aids in resisting colony collapse (Holmes, Tan, Wang, Oldryod and Beekman, 2015; Gloag, Tan, Wang et al., 2017).
3.2 Behavioural Patterns Apis cerana are social bees that nest in large hives. Apis cerana have very frequent absconding behaviour (Koetz, 2013). Due to lower stores of honey they are more dependent on resource availability and will disperse and start new colonies opportunistically. Apis cerana are also more prone to swarming regardless of resource availability of an area (Hepburn and Radloff, 2011: Heersink et al., 2016). The frequency of absconding and swarming varies depending on climate across Asia, it is more frequent in warm tropical areas and generally more frequent in comparison to A. mellifera (Hepburn and Radloff, 2011). Frequent absconding and swarming behaviour as well as fast brood development means A. cerana can disperse rapidly (Koetz, 2013). In general A. cerana are healthier than A. mellifera in the presence of the common honey bee diseases and parasites. Apis cerana have strong, hygienic grooming behaviours making them very effective at removing diseases (Lin, Page, Li, et al., 2016). Colonies are relatively resistant to Varroa mite (and by association Deformed Wing Virus, DWV), they have lower incidences of American Foul Brood and Nosema spp. infections too (Buchler, Drescher & Tornier, 1992; Hepburn and Radloff, 2011; Lin et al., 2016).
3.3 Habitat Apis cerana occupies a wide and varied range of habitats from tropical to sub-tropical latitudes, high to low altitudes, dry, humid to semi-desert climates as well as tropical climates (Koetz, 2013; Hepburn and Radloff, 2011). Across Asia A. cerana tends to prefer secondary forests, agricultural land, or disturbed areas (Koetz, 2013). However, it can colonise in primary forests too (Koetz, 2013). In Australia A. cerana tends to be found in open disturbed areas, but has been seen to make use of mangroves, and eucalyptic woodland (Koetz, 2013). Nests are mostly found between 2-10m high in tree hollows, crevices, caves, and house cavities (Koetz, 2013).
3.4 Foraging and Feeding Apis cerana are generalist pollen and nectar feeders utilising many species of flowering plants (Koetz, 2013). During foraging A. cerana prioritises the highest density resource available, which can be plants with many flowers or areas of a high density of flowering plants (Hepburn and Radloff, 2011). They are commonly found in agricultural crops and will target native species that flower in high density. Foraging times are generally earlier in the morning and have been recorded at temperatures of between 15.5°C-21°C (Rice, Schnieder, Barnett, & Cowen, 2016; Koetz, 2013). Differing foraging distances have been reported ranging from 250m-2500m from the hive with an average distance between 200m-900m (Hepburn and Radloff, 2011; Koetz, 2013).
3.5 Ecological Role and Uses in Native Range Apis cerana provides vital pollination services in Asia for both native and cultivated plants. Apis cerana is considered a better pollinator than A. mellifera due to its longer foraging period, ability to forage in lower temperatures, and their higher ratio of pollen collectors to nectar collectors (Koetz, 2013; Li, Qin, Wu et al., 2012). Apis cerana improve yields of agricultural fruit, vegetables, grains, nuts seeds and more throughout Asia (Koetz, 2013). Apis cerana also provides a food source for other species that thieve on the honey including a range of arthropods, birds, and mammals in their native range (Koetz, 2013).
4.0 Potential Impact 4.1 Native Species Diversity and Competition Little research has investigated the negative impacts of Apis and Bombus impacts on the New Zealand ecosystem compared to the positive impacts such as pollination. It is likely that there is some degree of competition for resources, such as nesting sites and nectar sources with native species like the Tui (Prosthemadera novaeseelandiae), Bellbird (Anthornis melanura), and native bee species to name a few, but this is poorly documented and therefore difficult to assess (Goulson, 2003). Native bees preferentially pollinate native plants while A. mellifera generally pollinate introduced species, but this is only a trend and not a niche division (Goulson, 2003; Early, 2007b). Given the lack of data we do need to err on the side of caution and not assume there will be no impact on native species particularly nectar feeding birds and invertebrates. However, given the very similar niche and interspecies competition in cohabited areas in Asia it is likely that A. cerana will share a similar dietary preference to A. mellifera in New Zealand. The presence of A. cerana will likely reduce numbers of A. mellifera, or vice versa, in areas ( Hepburn and Radloff, 2011; He et al., 2013) meaning overall honey bee pressure on other invertebrates and birds will probably not change to a significant extent. Because of this, A. cerana is not likely to have a large effect on New Zealand native species diversity as any significant impacts to the New Zealand ecosystem by the Apis genus would have occurred during the A. mellifera introduction 78 years ago (Early, 2007b). The main impact of an A. cerana incursion will be on A. mellifera and Bombus species (both introduced genus themselves) through direct competition for resources and disease spread (Pirk et al., 2017).
4.2 Ecosystem Change Establishment is likely to change plant spread and density within New Zealand, but it is difficult to determine how much (Goulson, 2003). A. cerana is considered a better pollinator than A. mellifera in Asia with increased pollination rates of both crop and native species (Dyer and Seeley, 1991; Hepburn and Radloff, 2011). Apis cerana has a stronger preference for high density feeding areas, therefore ratios of denser flowering plants and denser flowering areas in New Zealand may increase (Hepburn and Radloff, 2011). Apis cerana will likely impact the colder climate plant species as A. cerana is more cold tolerant than A. mellifera (Ruttner, 1988; Verma as cited in Koetz, 2013). This includes plants that flower earlier in the season, earlier in the morning, further south and at higher altitudes. Establishment could be beneficial in encouraging native plant spread as well as harmful by increasing introduced species spread (Butz Huryn, 1995). A.cerana has an affinity for disturbed and agricultural habitats so we would likely see an increased impact on weed species spread which will negatively impact on New Zealand native habitats and resource availability for our native species (Koetz, 2013). The agricultural industry may stand to benefit from an introduction of A. cerana due to their superior pollination rates (Li et al., 2012). New Zealand agriculture has a clear need for honey bee crop pollination which bee keepers are resistant to fill (MPI Apiculture monitoring program report, 2016). Wild populations of A. cerana could fill this void. Having more pollinator species diversity in New Zealand would also create a natural resistance in our pollinator community should a serious disease outbreak occur within one of those species like we are seeing now with Varroa and its impact on our European bees. However this is a double edged sword in that increased pollination rates can also mean increased plant disease spread. With issues such as Myrtle rust currently in New Zealand more plant visits by pollinators could be more devastating to our eco system. Other impacts that we may see are possible decreases in pollination rates due to intense interspecies competition in some areas (reducing time spent on each flower) by A. cerana and A. mellifera (Hepburn and Radloff, 2011; He et al., 2013).
4.3 New Zealand Honey Bee Competition Although A. mellifera in an introduced species, it has a very important role in New Zealand ecology and industry and any threat to this species should be taken seriously. There are 684,046 hives currently registered in New Zealand and one of the main issues put forth by Apiculture New Zealand was apiary site competition (Brown, 2017; MPI apiculture monitoring program, 2016). A. cerana behavioural and reproductive traits mean wild populations could possibly establish and spread fast. This increased competition from wild populations of bees will put more pressure on the apiary colonies. Numerous studies in Asia show that it is highly likely that there will be interspecies competition between A. cerana and A. mellifera (Hepburn and Radloff, 2011; He, Wang, Qin, et al., 2013; Koetz, 2013; Forsgren, Wei, Guiling et al., 2015). Evidence from Taiwan, Japan, Vietnam and China, is suggesting A. mellifera are tending to dominate and out-compete A. cerana (Koetz, 2013). When placed in close confinement the larger A. mellifera are stronger and more aggressive than A. cerana. Apis cerana shows avoidance by reducing foraging time in the presence of A. mellifera (Hepburn and Radloff, 2011, Koetz, 2013). Further observations of the recent Australian incursion support this (Koetz, 2013). However, a case study in the Solomon Islands showed different results. After the incursion in 2003 the A. mellifera population crashed. By 2008 there were only five hives (from 2000 originally) left on the main island. This was attributed to three reasons; 1-competition for floral resources, 2- A. cerana robbing A. mellifera hives, and 3-introduction of the Nosema ceranae pathogen (Koetz, 2013, Anderson, Annand, Lacey, Ete, 2012). It is now suspected that poor bee keeping practice and N. ceranae introduction may have played the largest part in the A. mellifera decline as new farming techniques and A. cerana baiting control have seen the resurgence of A. mellifera which is now able to coexist with A. cerana on the Solomon Islands (Anderson et al., 2012). Interspecific competition in New Zealand is not likely to directly cause extinction of A. mellifera but would likely reduce A. mellifera numbers in wild populations and cause some issues with farmed populations such as hive robbing and reducing pollen/nectar resources. It is hard to say which way an incursion will go but given the existing isolation of New Zealand honey bees lack of defence against A. cerana is a possibility.
4.4 New Zealand Honey Bee Disease Risk New Zealand Apis mellifera are highly vulnerable to disease. The Varroa mite invasion in 2000 and more recently N. Ceranae in 2010 are prominent examples of the susceptibility of New Zealand honey bees to novel diseases (Botias, Anderson, Meana, et al., 2012; Brown, 2017). Mechanisms of spread, once establishment occurs, could include interspecies hive invasions, interspecies mating, and pollen contamination (Singh, Levitt, Rajotte et al., 2010). Apis cerana have more hygienic colonies in general due to more intense grooming behaviours (Buchler et al, 1992; Li et al., 2012; Yanez, Zheng, Su et al., 2015). They could potentially provide disease resistance in wild populations that reduce pressure on European bee colonies from parasites and disease (Yanez et al., 2015; Lin et al., 2016; Pirk, C., Crewe, Moritz, & Nicolson, 2017). However, Asian bees pose very high risk of bringing in new diseases and parasites that could decimate New Zealand Apis (and Bombus) species (Li et al., 2012). Infection rates of various diseases across Asia vary greatly (from 0-98%) and there is no way of quickly and efficiently knowing which are carrying other pests (Li et al., 2012). Most Apis diseases and parasites developed in Asian bee populations and jumped to European bees. Diseases including Melissococcus plutonius which causes American foulbrood, the parasite Varroa destructor which can cause Deformed Wing Virus, and microsporidian parasites N. ceranae and N.apis (Chen and Huang, 2010; Forsgren and Fries, 2010; Li et al., 2012; Yanez et al., 2015). There are also a few instances of diseases transmitted from A. mellifera to A. cerana (Koetz, 2013; Hou, Li, Lou, Deng and Diao, 2016) as well as cross genus infections from Bombus to A. cerana (Li et al., 2012). In the Solomon Islands Varroa jacobsoni, a species which parasitizes A. cerana was found in an A. mellifera hive suggesting this species jump could be possible in a few years (Anderson et al., 2012). With the introduction of A. mellifera in Asia there is sufficient interspecies interaction encouraging species jump of diseases which could spread to New Zealand (Solignac, Cornuet, Vautrin et al., 2005; Navajas, Anderson, de Guzman et al., 2010). We also need to be very concerned about haplotypes of parasites and disease that have not yet left Asia. There is a genetic bottle neck between Asian and European honey bee populations that can cause devastating effects to New Zealand honey bees if transmitted via an Asian honey bee incursion. Only 2/8 haplotypes of Varroa destructor have spread out of Asia (Navajas et al., 2010). The other species V. jacobsoni has not invaded populations outside of Asia yet (Navajas et al., 2010).
4.5 Human Health and Uses Apis cerana are found to have a more docile temperament than A. mellifera meaning they pose less risk to people with allergies (Ruttner, 1988). They will typically hide rather that attack and have a low sting tendency which explains the dominance patterns of A. mellifera over A. cerana common across Asia (Ruttner, 1988; Koetz, 2013). Apis cerana also produce honey in commercially viable quantities albeit significantly less than A. mellifera (2-5kg/hive compared to 29.1kg/hive in New Zealand in 2016 respectively) (Hepburn and Radloff, 2011; MPI Apiculture Monitoring Program, 2016). However, given the low yields, likely adoption of A. cerana for honey production in New Zealand is low and the disease risk that they could pose to A. mellifera could mean that A. mellifera may require heavier pesticide use in future should they contract deadly diseases from A. cerana. Generally A. cerana don’t appear to pose any great threat to humans as they have lived alongside humans in Asia for many years without major issues but could have knock on effects to New Zealand bees.
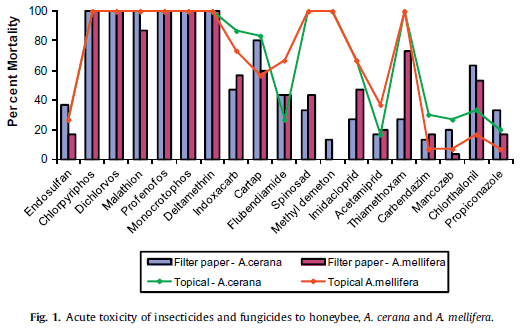
5.0 Control Technologies and Strategies 5.1 Potential Eradication Apis cerana has environmental plasticity as well as fast colonising biology. Eradication would need to be carried out quickly and early in the establishment phase if it was to be successful. Efforts in Cairns, Australia in 2007 failed because of three reasons; frequent relocations due to high sensitivity to change, their rapid breeding and long travel distances, and limitation of surveillance methods to locate nests (Australian Government Department of Agriculture and Water Resources, 2016). Apis cerana is sensitive to the same pesticides as A. mellifera so any chemical eradication later on would detrimentally effect A. mellifera populations (Stanley, Sah, Jain, Bhatt, Sushil, 2015; Yasuda, Sakamoto, Goka et al., 2017). See Figure 8 for a full list of poisons and their toxicity to A. cerana. Organophosphates have the most toxic effect but affect both A. cerana and A. mellifera (Stanley et al., 2015). During eradication all queen bees will need to be located and destroyed and surrounding areas must be thoroughly checked for an extended period of time after for absconded colonies and colonies reproducing by thelytoky. If establishment and spread of A. cerana was found in New Zealand control techniques will need to be reassessed. Eradication at this point could have severe effects on the native ecosystem as well as the New Zealand honey industry and a switch to monitoring and bait control may be more economically feasible.
Figure 8. Acute toxicity of insecticides and fungicides on Apis cerana (Asian honey bee) and Apis Mellifera (European honey bee). Poisons were administered via topical administration to the bee or by soaking filter paper in the poison and allowing the bee to contact the filter paper. Image sourced from Stanley et al., 2015.
5.2 Monitoring Effective detection methods in Australia that are applicable in New Zealand are floral surveillance methods involving ‘targeted floral surveillance’ or ‘Timed or limited floral surveillance’ (Rice et al., 2016). A full description of these methods can be found in the Australian Government: Department of Agriculture and Water Resources surveillance manual at http://www.agriculture.gov.au/pests-diseases-weeds/bees/the-asian-honey-bee-in-australia . In the Solomon islands the easiest and most cost effective lour used is a flat open dish containing honey and honey comb collected from wild Asian bee colony's which is then later replaced with 60% sugar syrup. When offered early in the morning before the European bees were foraging (9am) it attracted no non target pests in the Solomon Islands (Anderson et al., 2012). This could be effective in New Zealand as well.
5.3 Baiting and Control There is a lack of research on control strategies for A. cerana since it is has only become invasive to the Solomon islands and Australia. However, control efforts in the Solomon Islands are proving effective in suppressing A. cerana populations. Their methods involve 1ltr of 0.05% Fipronil in sugar syrup supplied at one feeding station over 60 minutes. This was found to suppress A. cerana over 0.5km² for up to 8 months (Anderson et al., 2012). European bees must be removed from the area to a minimum 5km away during baiting and only returned to the area after 2 weeks (Anderson et al., 2012). It is yet to be known if this would be effective in New Zealand too given the different geography. This technique would need to be adopted by the bee keepers themselves and strict regulations would need to be put in place to avoid damage to other business in surrounding areas such as orchards, farms, and other bee keepers.
5.4 Biological control Natural predators of A. cerana that could exist in New Zealand include wasps, hornets, and ants (Hepburn and Radloff, 2011; Australian Government Department of Agriculture and Water Resources, 2016). In New Zealand Argentine ants may be an issue for A. cerana however are not likely to have much effect due to A. cerana’s frequent absconding behaviour. Other natural predators have been ruled out for this reason too and because significant predation has not been seen in A. mellifera. There are no known host specific predators that could be introduced to New Zealand for A. cerana biocontrol that wouldn't come with a range of other risk factors. Known diseases and parasites common in Asian populations also come with too many risks as there is a history of multiple species jumps between A. cerana and A. mellifera within Asia since the introduction of A. mellifera to Asia and this could be extremely damaging to the honey and agricultural crop industry (Botias et al., 2012; Forsgren et al., 2015).
6.0 Knowledge Gaps and Research Recommendations 6.1 Knowledge Gaps What is apparent in the literature is a bias in research focused on the positive effects of honey bees in general, no doubt due to majority funding by stake holders in the honey industry. There are large knowledge gaps on the negative effects of honey bees on New Zealand species, particularly regarding competition for nest sites, competition for food resources (pollen and nectar), and interspecies aggression/defence. Further research also needs to be done into interspecific competition between invasive A. cerana and A. mellifera. Most research to date is centred on Asian populations where A. cerana is the native species and A. mellifera the invasive. The Solomon Islands and Australia are the only two countries where A. cerana is viewed as a pest and although species collapse was observed in the Solomon Islands with A. cerana establishment, the confounding variables around the collapse including poor bee keeping management and simultaneous disease introduction, hint that the bees themselves may not have that strong an impact. Other areas of interest where knowledge is lacking are the species specific interactions between A. cerana and New Zealand species of plants and animals. Although, based on similar niches with A. mellifera, we can make estimations of diet and habitat range we cannot be certain if A. cerana will be limited by the same biotic and abiotic factors as A. mellifera in New Zealand.
6.2 Research Recommendations There are already response programs in place in Australia and the Solomon Islands that are proving effective and can provide a model for control in New Zealand. Threat of incursion levels is still reasonable at this point therefore the main research efforts should include the following:
Research that will be required should an incursion threat increase to highly likely:
6.4 Justification Against Inclusion There could be a few positive benefits to allowing A. cerana establishment in New Zealand. It is highly likely that A. cerana will increase pollination rates among agricultural crops, and bring more species diversity into our pollinators. This would create greater protection against disease outbreaks which could have debilitating impacts on our agriculture sector, and bring great economical value to industry. Apis cerana is a relatively hygienic bee with good disease resistance. It is generally dominated by A. mellifera in interspecies conflict and is generally more timid and stings less. For these reasons they could be considered to help with disease management in wild honey bee populations in New Zealand by keeping disease levels lower than A. mellifera is capable of achieving.
6.3 Justification for Inclusion Likeliness of establishment is extremely high based on the climate model and model species (A. mellifera) present in New Zealand. It is also a generalist pollinator/nectar feeder displaying immense plasticity in its native range (Hepburn and Radloff, 2011). Previous attempts at eradication have failed due to high absconding and swarming behaviour. An Incursion in New Zealand is very likely to lead to establishment within a short period of time, after which eradication will be unlikely (Anderson et al., 2012; Australian Government Department of Agriculture and Water Resources, 2016).
An Apis cerana incursion will affect four main areas in New Zealand:
As knowledge in this area is limited we must err on the side of caution regarding estimations. For native bees and pollinator species we can, at best, expect no impact from A. cerana and, at worst, anticipate a negative impact on these species which reduces native species population numbers and possible extinction (Goulson, 2003).
There will most likely be small changes in native ecosystem plant distributions over time but also an increase in introduced plant pollination and spread. It is more likely that agricultural plants and introduced weeds will be prioritised over native species (Butz Huryn,1995).
The apiculture industry will receive most of the negative impacts of an incursion because of the high risk of disease and potential resource competition Apis cerana brings with it (Hepburn and Radloff, 2011). Utilising Apis cerana in their business is also unlikely due to the low yields of honey produced and frequent absconding behaviour. Wild populations of A. cerana could also encroach on ‘for hire’ pollination services.
New Zealand agriculture could gain some benefit from A. cerana with increased natural pollination services however, if A. cerana negatively affected A. mellifera populations with disease and caused rapid decline of A. mellifera populations this would not be the case.
6.5 Conclusion Apis cerana is a generalist pollinator that has remarkable plasticity. There is ample evidence to suggest it can survive comfortably in New Zealand and will most likely encroach on the A. Mellifera niche. The likelihood of running a successful eradication program once this species establishes is very low. It is important to take steps in minimising the possibility of an incursion and contingency plans should be in place if this event were to occur. The risks to the natural ecosystem and industry are high and the benefits small. Early eradication is a far more cost effective measure in the long run as it is not likely to succeed later in the establishment phase. If establishment in New Zealand occurs, ongoing monitoring and control may be required indefinitely. Action on this species needs to be at the boarder focusing on the ports and maritime traffic from the North Asia and South East Asian region. For these reasons we are recommending its inclusion as a monitored pest. References
Anderson, D.; Annand, N.; Lacey, M.; & Ete, S. (2012). Control of Asian Honey Bees in Solomon Islands; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia.
Australian Government Department of Agriculture and Water Resources (2016, August). Status of the Asian honey bee in mainland Australia. Retrieved 15/5/17 from http://www.agriculture.gov.au/pests- diseases-weeds/bees/the-asian-honey-bee-in-australia.
Botias, C., Anderson, D. L., Meana, A., Garrido-Bailon, E., Martin-Hernandez, R., & Higes, M. (2012). Further evidence of an oriental origin for Nosema ceranae (Microsporidia: Nosematidae). J Invertebr Pathol, 110(1), 108-113. doi:10.1016/j.jip.2012.02.014
Brown, P. (2017). New Zealand colony loss survey report-2016. Ministry for Primary Industries Report 2253- 3923. Retrieved from MPI News and Resources, publications database.
Buchler, R., Drescher W., & Tornier, I. (1992) Grooming behaviour of Apis cerana, Apis mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Experimental & Applied Acarology, 16, 313-319.
Butz Huryn, V. M. (1995). Use of native New Zealand plants by honey bees (Apis mellifera L.): A review. New Zealand Journal of Botany, 33(4), 497-512. doi:10.1080/0028825x.1995.10410621
Chen, Y. P., & Huang, Z. Y. (2010). Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie, 41(3), 364-374. doi:10.1051/apido/2010021
Dyer, F., & Seeley, T. (1991). Behavior and the evolution of worker tempo in four honey bee species. Ecology, 72(1), 156-170.
Early, J. (2007a, September). 'Wasps and bees - Native bees', Te Ara - the Encyclopedia of New Zealand. Retrieved 15 May 2017 from http://www.TeAra.govt.nz/en/wasps-and-bees/page-4
Early, J. (2007b, September). 'Wasps and bees – Introduced bees', Te Ara - the Encyclopedia of New Zealand. Retrieved 15 May 2017 from http://www.TeAra.govt.nz/en/wasps-and-bees/page-5
Forsgren, E., & Fries, I. (2010). Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet Parasitol, 170(3-4), 212-217. doi:10.1016/j.vetpar.2010.02.010
Forsgren, E., Wei, S., Guiling, D., Zhiguang, L., Van Tran, T., Tang, P. T., . . . Fries, I. (2015). Preliminary observations on possible pathogen spill-over from Apis mellifera to Apis cerana. Apidologie, 46(3), 265-275. doi:10.1007/s13592-014-0320-3
Gloag, R., Tan, K., Wang, Y., Song, W., Luo, W., Buchman, G., . . . Oldroyd, B. P. (2017). No evidence of queen thelytoky following interspecific crosses of the honey bees Apis cerana and Apis mellifera. Insectes Sociaux, 64(2), 241-246. doi:10.1007/s00040-016-0538-3
Goulson, D. (2003). Effects of Introduced Bees on Native Ecosystems. Annual Review of Ecology, Evolution, and Systematics, 34(1), 1-26. doi:10.1146/annurev.ecolsys.34.011802.132355
He, X., Wang, W., Qin, Q., Zeng, Z., Zhang, S., & Barron, A. B. (2012). Assessment of flight activity and homing ability in Asian and European honey bee species, Apis cerana and Apis mellifera, measured with radio frequency tags. Apidologie, 44(1), 38-51. doi:10.1007/s13592-012-0156-7
Heersink, D. K., Caley, P., Paini, D. R., & Barry, S. C. (2016). Quantifying the Establishment Likelihood of Invasive Alien Species Introductions Through Ports with Application to Honeybees in Australia. Risk Anal, 36(5), 892-903. doi:10.1111/risa.12476
Hepburn, R., Radloff, S. (2011). Honeybees of Asia. London New York: Springer Heidelberg Dordrecht
Holmes, M. J., Tan, K., Wang, Z., Oldroyd, B. P., & Beekman, M. (2015). Genetic reincarnation of workers as queens in the Eastern honeybee Apis cerana. Heredity (Edinb), 114(1), 65-68. doi:10.1038/hdy.2014.70
Hou, C., Li, B., Luo, Y., Deng, S., & Diao, Q. (2016). First detection of Apis mellifera filamentous virus in Apis cerana cerana in China. J Invertebr Pathol, 138, 112-115. doi:10.1016/j.jip.2016.06.011
Koetz, A. H. (2013). Ecology, Behaviour and Control of Apis cerana with a Focus on Relevance to the Australian Incursion. Insects, 4(4), 558-592. doi:10.3390/insects4040558
Li, J., Qin, H., Wu, J., Sadd, B. M., Wang, X., Evans, J. D., . . . Chen, Y. (2012). The prevalence of parasites and pathogens in Asian honeybees Apis cerana in China. PLoS One, 7(11), e47955. doi:10.1371/journal.pone.0047955
Ministry for Primary Industries. (2017). Ministry for Primary Industries 2016 apiculture monitoring programme. Ministry of Primary Industries Report 978-1-77665-487-1. Retrieved from MPI News and Resources, publications database.
Lin, Z., Page, P., Li, L., Qin, Y., Zhang, Y., Hu, F., . . . Dietemann, V. (2016). Go East for Better Honey Bee Health: Apis cerana Is Faster at Hygienic Behavior than A. mellifera. PLoS One, 11(9), e0162647. doi:10.1371/journal.pone.0162647
Navajas, M., Anderson, D. L., de Guzman, L. I., Huang, Z. Y., Clement, J., Zhou, T., & Le Conte, Y. (2009). New Asian types of Varroa destructor: a potential new threat for world apiculture. Apidologie, 41(2), 181-193. doi:10.1051/apido/2009068
Pirk, C. W. W., Crewe, R. M., Moritz, R. F. A., & Nicolson, S. (2017). Risks and benefits of the biological interface between managed and wild bee pollinators. Functional Ecology, 31(1), 47-55. doi:10.1111/1365-2435.12768
Queensland Government Department of Agriculture and Fisheries (2016, December). Asian honey bees in Queensland. Retrieved 15/5/17 from https://www.daf.qld.gov.au/animal- industries/bees/diseases-and-pests/asian-honey-bees
Rice, A, Schneider, I, Barnett, S & Cowan, S (2016). NAQS Asian honey bee floral surveillance manual, Northern Australia Quarantine Service, Department of Agriculture and Water Resources, Canberra. CC BY 3.0.
Ruttner, F. (1988). Apis cerana Fabricius 1793:327, In Ruttner, F. (Eds), Biogeography and Taxonomy of Honeybees. (pp. 120-160). Heidelberg, Germany: Springer-Verlag Berlin.
Singh, R., Levitt, A. L., Rajotte, E. G., Holmes, E. C., Ostiguy, N., Vanengelsdorp, D., . . . Cox-Foster, D. L. (2010). RNA viruses in hymenopteran pollinators: evidence of inter-Taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS One, 5(12), e14357. doi:10.1371/journal.pone.0014357
Solignac, M., Cornuet, J., Vautrin, D., Le Conte, Y., Anderson, D., Evans, J., C., . . . Navajas, M. (2005) The invasive Russian and Japanese types of Varroa destructor, ectoparasite mite of the Western honey bee (Apis mellifera), are two partially isolated clones, Proc. R. Soc., 272, 411–419.
Stanley, J., Sah, K., Jain, S. K., Bhatt, J. C., & Sushil, S. N. (2015). Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere, 119, 668-674. doi:10.1016/j.chemosphere.2014.07.039
Tan, K., Qu, Y., Wang, Z., Liu, Z., & Engel, M. S. (2015). Haplotype diversity and genetic similarity among populations of the Eastern honey bee from Himalaya-Southwest China and Nepal (Hymenoptera: Apidae). Apidologie, 47(2), 197-205. doi:10.1007/s13592-015-0390-x
Yañez, O., Zheng, H.-Q., Su, X.-L., Hu, F.-L., Neumann, P., & Dietemann, V. (2016). Potential for virus transfer between the honey bees Apis mellifera and A. cerana. Journal of Apicultural Research, 54(3), 179-191. doi:10.1080/00218839.2015.1128145
Yasuda, M., Sakamoto, Y., Goka, K., Nagamitsu, T., & Taki, H. (2017). Insecticide Susceptibility in Asian Honey Bees (Apis cerana (Hymenoptera: Apidae)) and Implications for Wild Honey Bees in Asia. J Econ Entomol, 110(2), 447-452. doi:10.1093/jee/tox032 Apis cerana is a generalist pollinator that has remarkable plasticity. There is ample evidence to suggest it can survive comfortably in New Zealand and will most likely encroach on the A. Mellifera niche. The likelihood of running a successful eradication program once this species establishes is very low. It is important to take< |