AWA: Academic Writing at Auckland
Title: Scientific writing skills practice
|
Copyright: Hadassah Patchigalla
|
Description: Introduction for a research essay on a drug (Zopiclone).
Warning: This paper cannot be copied and used in your own assignment; this is plagiarism. Copied sections will be identified by Turnitin and penalties will apply. Please refer to the University's Academic Integrity resource and policies on Academic Integrity and Copyright.
|
Writing features
|
Scientific writing skills practice
Part A: IntroductionZopiclone is a sleep-inducing drug developed by Rhône-Poulenc, after around 12 years of research aiming to find a new class of drugs that were pharmacologically similar to the benzodiazepines (1). Rhône-Poulenc was a French pharmaceutical company that is now under Sanofi-Aventis – currently the main manufacturer of Zopiclone – which is also commonly known as Imovane and Zimovane (2). Zopiclone was the first drug in the cyclopyrrolone family: the new class of hypnotics discovered in the 1980s which were structurally distinct from, but acted like, benzodiazepines (3). A new family of drugs was sought after to replace benzodiazepines as they had a variety of unfavourable side effects during the day, such as drowsiness, withdrawal effects and dependence (4, 5). Zopiclone has an agonist effect on type A γ-aminobutyric acid receptors (GABAA receptors), binding to the same site as benzodiazepines (2, 3). The five characteristic activities exhibited by benzodiazepines are also properties of Zopiclone; it has anticonvulsant, muscle-relaxant, anti-anxiety, sedative and hypnotic effects (1, 6). Despite the wide range of properties Zopiclone has, it is primarily administered to treat transient, short-term and chronic insomnia (3, 6). It can be used as a pre-operative sedative for hospitalised patients, but other drugs such as flurazepam and diazepam (benzodiazepines), are more suitable for this use as they are more effective at alleviating preoperative anxiety (3). Zopiclone does not tend to produce the adverse effects seen as a result of benzodiazepine use for insomnia treatment. Difficulty waking and reduced morning concentration are not common, and the drug does not have a high potential for dependence (5). It can be used on a wide variety of people, both elderly and younger patients tolerate Zopiclone well; however, it is not recommended for use by pregnant women as there are no sufficiently large enough studies confirming its safety during pregnancy (5, 7). An adverse effect of Zopiclone is a bitter aftertaste, but this is not relatively frequent among users (5).
Part B: Graphing exercise
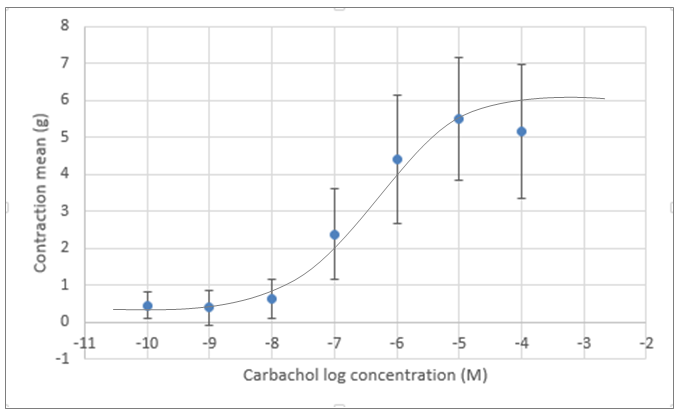
Figure 1: Mean contraction of smooth muscle of guinea pig ileum in response to increasing concentrations of an acetylcholine analogue (carbachol). Concentrations of carbachol increased ten-fold for each of the seven data points measured (starting at 1 x 10-10 M and increased up to 1 x 10-4 M). Data were graphed using a log scale of the carbachol concentration to observe the sigmoidal curve. The error bars represent the standard deviations of the mean contraction observed.
Part C: Referencing exerciseReferences Julou L, Bardone MC, Blanchard JC, Garret C, Stutzmann Pharmacological studies on Zopiclone. Pharmacology 1983; 27 (2): 46-58 Curreen M, Lidmila Zopiclone: Is there cause for concern in addiction services and general practice? Int J Risk Saf Med 2014; 26(4): 183-9 Goa KL, Heel RC. Zopiclone A review of its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Efficacy as an Drugs 1986; 32(1): 48-65 Wheatley New Hypnotic Agents: Clinical Studies in General Practice. Pharmacol Biochem Behav 1988; 29(4): 811-13 Noble S, Langtry H, Lam Zopiclone: an update of its Pharmacology, Clinical Efficacy and Tolerability in the Treatment of Insomnia. Drugs 1998, February; 55(2): 277-302 Sanofi-Aventis New Zealand Imovane Tablets. Data Sheet (2015). Available at www.medsafe.govt.nz/profs/datasheet/i/Imovanetab.pdf Diav-Citrin O, Okotere B, Lucarelli K, Koren Pregnancy outcome following first- trimester exposure to zopiclone: a controlled cohort study. Am J Perinatol 1999; 16(4): 157-60 Dogrell SA. Differential antagonism of the initial fast and secondary slow contractile responses of the rate isolated aorta to 5-hydroxytryptamine by mianserin and J Auton Pharmacol 1987; 7(2): 157-64 Pistilli MJ, Petrik JJ, Holloway AC, Crankshaw D Immunohistochemical and functional studies on calcium-sensing receptors in rate uterine smooth muscle. Clin Exp Pharmacol Physiol Supp 2012; 39(1): 37-42 Zhang X, Zang N, Wei Y, Yin J, Teng R, Seftel A, et Testosterone regulates smooth muscle contractile pathways in rat prostate: emphasis on PDE5 signalling. Am J Physiol Endocrinol Metab 2012; 302(2): 243-53 Gratzke C, Ückert S, Kedia G, Reich O, Schlenker B, Seitz M, et In vitro effects of PDE5 inhibitors sildenafil, vardenafil and tadalafil on isolated human uteral smooth muscle: a basic research approach. Urol Res 2007; 35(1): 49-54 Rang Stimulant Actions of Volatile Anaesthetics on Smooth Muscle. Brit J Pharmacol 1964; 22: 356-65 Danish Myo Tissue Organ Bath System Model 750TOBS with Data acquisition software. User Manual Version 2.2 (2008). Available at http://www.dmt.dk/files/manualer/manual_750tobs.pdf Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, et Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3): S729-67 Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson Rang and Dale’s Pharmacology. 7th ed. London: Elsevier Churchill Livingstone; 2012 Glass M, Anderson L, Paxton J, Tingle M, Holford N, Graham S, et al. Drug Targets 3. In: Medsci 204 Course Manual [lecture notes]. Auckland: University of Auckland; 2015. p 35 |
|