AWA: Academic Writing at Auckland
Title: Agent H3 as a Potential Replacement Fire Extinguishant to Halon 1301
|
Copyright: Jonathan Kah
|
Description: The assignment is designed to give you practice in developing and publishing a technical report. Consider: Format and presentation; structure and content; style of writing; summary; references; figures. You are given some notes about the experiment; you are to write these up in the form of a technical report.
Warning: This paper cannot be copied and used in your own assignment; this is plagiarism. Copied sections will be identified by Turnitin and penalties will apply. Please refer to the University's Academic Integrity resource and policies on Academic Integrity and Copyright.
Agent H3 as a Potential Replacement Fire Extinguishant to Halon 1301
|
Agent H3 as a Potential Replacement Fire Extinguishant to Halon 1301 Sentinel Fire Protection Limited
Jonathan Kah 17th August 2017
Executive SummaryThis report provides an investigation into the testing of a fire suppression agent, Agent H3. The main purpose of the investigation is to determine whether Agent H3 will be a suitable replacement CFC (chlorofluorocarbon) free agent to the current industry standard Halon 1301, which contains CFCs. CFCs react in the atmosphere to destroy the ozone layer. The tests conducted in this investigation measures the effectiveness of Agent H3 as a fire suppressant used on a fire in an aircraft cargo container, simulating the conditions in a wide-body aircraft cargo hold. The data obtained was then compared to data obtained from a similar test using Halon 1301, to determine whether the new product would be more effective. The comparisons made were against the temperature acceptance, and the maximum temperature/time area criteria. These criteria measure the ability of the agent to knock down and extinguish a standard fire when it is discharged one minute after the temperature of the hold reaches 100°C. Agent H3 passed the maximum temperature criterion, and did not pass the area criterion. However, the measured value of the area was only slightly above that of Halon 1301. Since only six simulations were carried out, this value may not be representative of the true properties of Agent H3. Therefore, further testing is recommended for the agent, including more area tests, corrosion, toxicity, and by- product tests.
Table of Contents
Table of Figures Figure 1: Chlorofluorocarbon atomic arrangement................................................................................ 1 Figure 2: Simulated cargo compartment layout.................................................................................... 2 Figure 3: Layout of LD-3 containers.................................................................................................... 2 Figure 6: Maximum area under temperature-time graph by simulation................................................... 4
Table of Tables
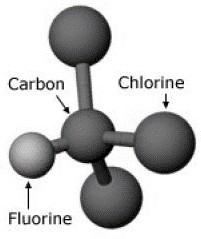
1. IntroductionIn the 1960s, the fire protection industry began installing halon extinguishers, the most widely used being the agent Halon 1301. It is effective at extinguishing a variety of fires, such as flammable liquids, electronics, and common combustibles. Halon 1301 is also only used in small quantities and thus has been deemed safe for use in human occupied spaces such as flight decks and passenger cabins. Furthermore, it has noncorrosive properties, doesn’t conduct electricity, and leaves no residue. This renders it an ideal agent for the required application being investigated in this report: a suppressant to be applied to a fire in a simulated wide-body cargo hold. This agent has been used extensively in both aircraft structure and equipment, with no other replacement agent meeting all the necessary requirements in aircraft fire protection. However, there is a major problem with the continued use of Halon 1301. The agent contains CFCs (chlorofluorocarbons, see Figure 1), which are chemicals that have an extremely long life. This has great implications in the upper atmosphere, where these chemicals react with the ultraviolet light, resulting in the damage to the ozone layer. An international treaty was established in 1987, known as the Montreal Protocol. It details the reduced use, and phasing out of production of CFCs. This had a great impact on the global use of Halon 1301 and other CFC containing chemicals. The 1992 London amendment gave exemptions for applications where there were no available alternatives to CFC based chemicals. Since the aviation industry had no replacement agent that met all the safety regulations, they have continued the use of Halon 1301 in aircraft (UNEP, 2006). For a replacement agent to be considered feasible, it must pass two acceptance criteria. These are the temperature acceptance criterion and the maximum temperature/time area criterion, which are a measure of the ability of the agent to suppress and extinguish a fire. To be considered, the test values of the replacement agent must be equal to or less than those obtained from the earlier Halon 1301 tests. In addition to these criteria, the replacement agent must not damage aircraft structural members, fittings or wiring, and must be non-toxic both in storage, and after extinguishing the fire (Bennet, 2011). This report details a method of testing that engineers at Sentinel Fire Protection Limited have executed on a potential replacement called Agent H3.
Figure 1: Chlorofluorocarbon atomic arrangement
2. Methods2.1 Cargo Compartment Layout
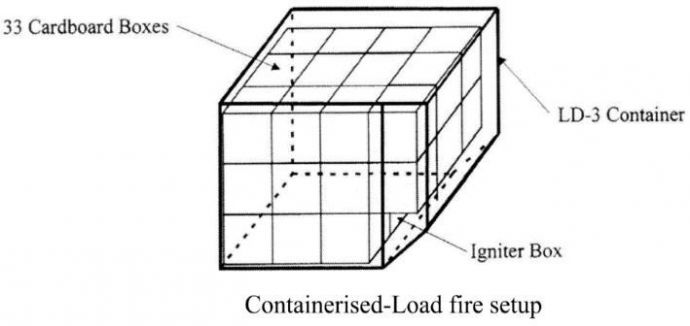
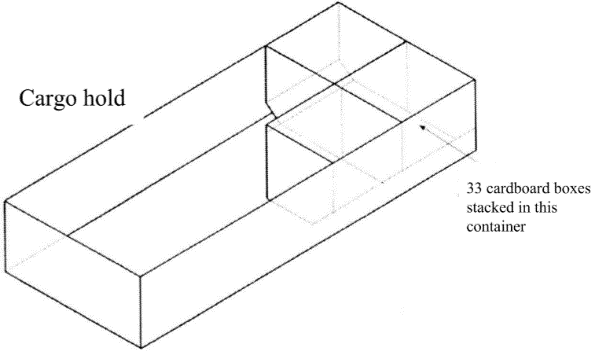
The fire scenario simulated was a containerised-load bulk fire. 33 single-wall corrugated square boxes (side length 450mm) were stacked in three layers, four tiers deep, in a LD-3 container of volume 58m3. The boxes were packed to touch each other to eliminate any significant air gaps between adjacent boxes. See Figure 2. Type K thermocouples were placed at 1.25m intervals along the ceiling of the simulation environment, protruding by 25mm. Two additional LD-3 containers were stored adjacent to the one containing the boxes. See Figure 3.
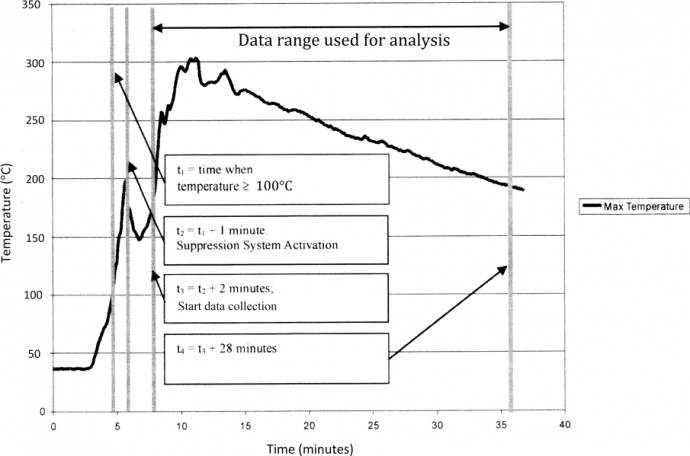
2.2 MethodFirstly, 33 boxes each containing 1.5kg of loosely packed 80gm/m2 standard shredded office copier paper, were packed into the simulation area. The boxes were then left for 24 hours ensure they were at standard room temperature and low humidity. The gas analyser was switched on and allowed to settle at this time. The igniter was placed in the centre of the outside box in the bottom layer of the second tier. See Figure 2. The igniter consisted of 2m nichrome wire with approximately 7ohm resistance, wrapped around four paper towels. Once the readout board for the thermocouples was checked, and all thermocouples verified to be working, a data-logger was connected to the readout board. This produces the graphs of temperature versus time; a typical output is shown in Figure 4. After this, the test timer was checked for satisfactory operation and zeroed. Type K thermocouples operate over a large range of temperatures (270 – 1260°C), which is ideal for this simulation (Thermometrics Corporation, 2012). The test was then carried out. The fire was ignited by running a 240V AC current to the igniter wire. The first recorded data point was t1, the time at which the temperature in the hold reached 100°C. Then at t2 (t1 + 1 minute), the fire suppression system was activated, releasing the extinguishant Agent H3 into the simulation cargo compartment. Data was collected from the thermocouples at 1 minute intervals from time t3 (t2 + 2 minutes) until t4 (t3 + 28 minutes). This process was repeated another five times, to obtain six data sets in total. These results have been entered into the table Table 1.
Figure 4: Generic time/temperature graph representing the output of one thermocouple test 3. ResultsThe results obtained from the thermocouples over the six tests were used to produce Table 1, and Figures 5 and 6. The standard deviation of all tests 1-6 were calculated for both the maximum temperature and the area. These were added to the respective maximum value of the 6 tests to give the numerical value to measure against the criteria. For Agent H3 to satisfactorily meet aviation standards, the summed values should be less than or equal to those of Halon 1301, as shown in the bottom row of Table 1.
Table 1: Results of the simulation
Consider the results above. The sum of the standard deviation and the maximum value for the maximum temperature for Agent H3 is lower than Halon 1301 – the maximum temperature criterion is passed. However, the sum for the area for Agent H3 is slightly larger than Halon 1301 – hence the area criterion is not met.
Figure 5: Maximum temperature by simulation
Figure 6: Maximum area under temperature-time graph by simulation
4. Conclusions and RecommendationsThis report details the methods and results of the testing of Agent H3, a potential replacement fire extinguishant to Halon 1301.
Further testing is recommended for Agent H3. Since only six simulations were conducted, the values may not be representative of the true properties of Agent H3. Corrosion, toxicity, and by-product tests must also be carried out and passed for Agent H3 to be approved as a fire suppressant for aircraft. 5. ReferencesBennet, R. (2011). Replacing halon in fire protection systems: A progress report. Aero Magazine, 4(44), 13-17.
Thermometrics Corporation. (2012). Type K thermocouples, data sheets. Retrieved from http://www.thermometricscorp.com/thertypk.html
UNEP. (2006). Handbook for the montreal protocol on substances that deplete the ozone layer. United Nations Environment Programme, |
|||||||||||||||||||||||||||||||||||||